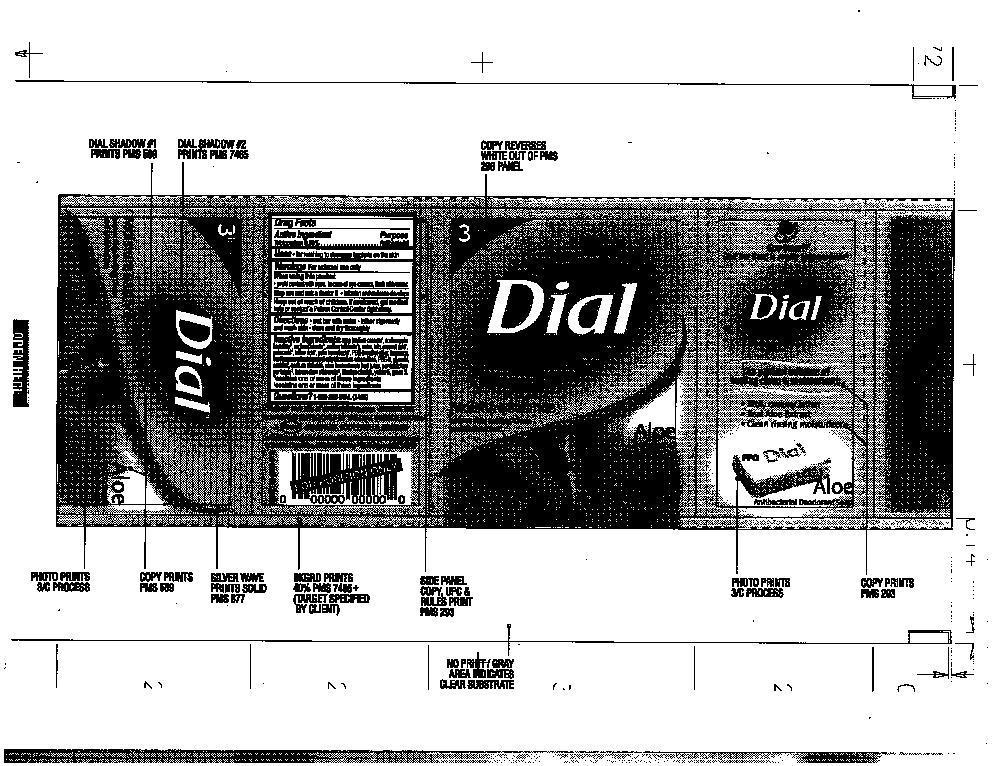
WARNINGS
For external use only
When using this product
Avoid contact with eyes. In case of eye contact, flush with water
Stop using and ask doctor if irritation and redness develops
INACTIVE INGREDIENTS
SOAP, (SODIUM COCOATE*, SODIUM PALM KERNELATE*, SODIUM PALMATE*, SODIUM TALLOWATE*), WATER, TALC, COCONUT ACID*, PALM ACID*, TALLOW ACID, PALM KERNEL ACID*, PEG-G METHYL ETHER, FRAGRANCE, SUNFLOWERSEEDAMIDOPROPYL ETHYLDIMONIUM ETHOSULFATE, PEG-9, GLYCERIN, SORBITOL, SODIUM CHLORIDE, ALOE BARBADENSIS LEAF JUICE, PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE, TITANIUM DIOXIDE, YELLOW 5, GREEN 3.