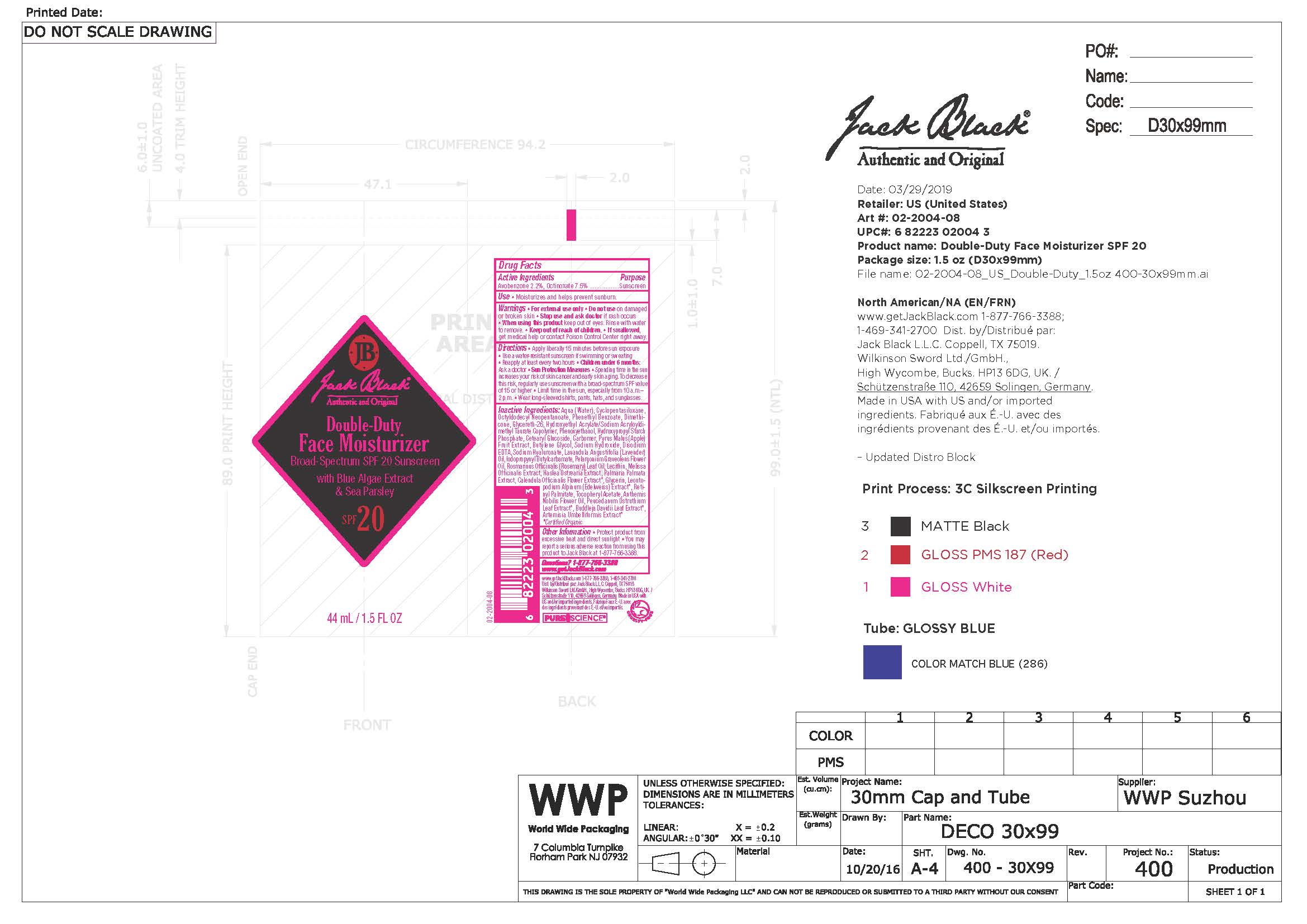
Inactive Ingredients
Purified Water, Cyclopentasiloxane, Octyldodecyl Neopentanoate, Phenylethyl Benzoate, Dimethicone, Glycereth-26, Hydroxyethyl Acrylate / Sodium Acryloyl Dimethyl Taurate Copolymer, Phenoxyethanol, Hydroxypropyl Starch Phosphate, Cetearyl Glucoside, Carbomer, Pyrus Malus (Apple) Fruit Extract, Butylene Glycol, Sodium Hydroxide, Disodium EDTA, Sodium Hyaluronate, Lavendula Angustifolia (Lavender) Oil, Iodopropynyl Butylcarbamate, Pelargonium Graveolens (Geranium) Flower Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Lecithin, Melissa Officinalis (Balm Mint) Extract, Haslea Ostreaia (Blue Algae) Extract, Palmaria Palmata (Sea Parsley) Extract, Calendula Officinalis (Calendula) Flower Extract, Glycerin, Leontopodium Alöinum (Edelweiss) Extract, Artemisia Umbelliformis Extract, Buddleja Davidii Leaf Extract, Peucedanum Ostruthium Leaf Extract, Tocopheryl Acetate, Retinyl Palmitate, Anthemis Nobilis (Roman Chamomile) Flower Oil
Warnings
- For external use only
- Do not use on damaged or broken skin
- Stop use and ask doctor if rash occurs
- When using this product keep out of eyes. Rinse with water to remove
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resisitant sunscreen if swimming or sweating
- Reapply at least every two hours
- Children under 6 months: ask a doctor.
- Sun Protection Measures:
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, use sunscreen with a broad spectrum SPF15 or higher
- Limit time in the sun, especxially from 10 a.m. - 2 p.m.
- Wear long sleeve shirts, pants, hats and sunglasses.