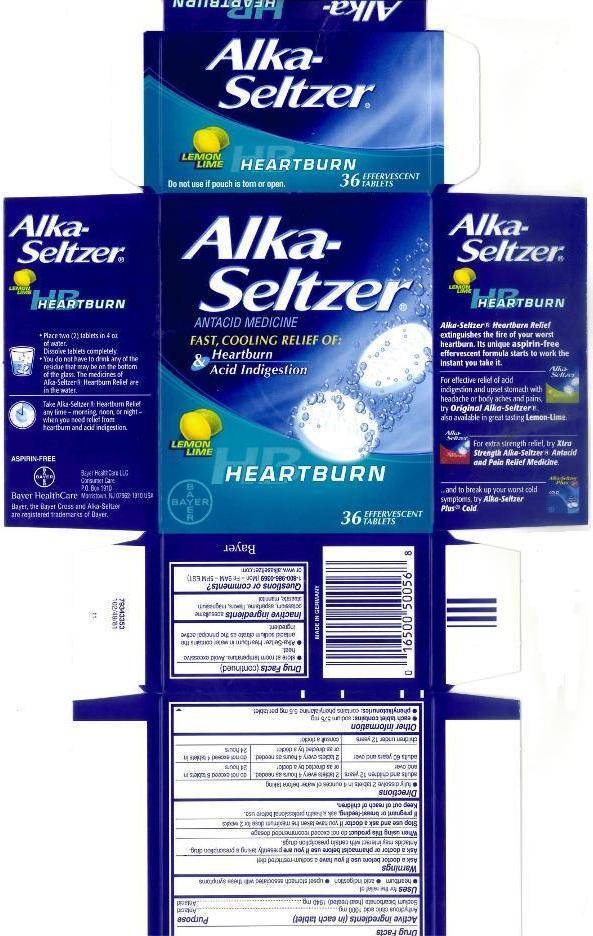
ALKA-SELTZER HEARTBURN- anhydrous citric acid and sodium bicarbonate tablet, effervescent
Bayer HealthCare LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Alka-
Seltzer
®
HEARTBURN
Warnings
Directions
- fully dissolve 2 tablets in 4 ounces of water before taking
| adults and children 12 years and over | 2 tablets every 4 hours as needed, or as directed by a doctor | do not exceed 8 tablets in 24 hours |
| adults 60 years and over | 2 tablets every 4 hours as needed, or as directed by a doctor | do not exceed 4 tablets in 24 hours |
| children under 12 years | consult a doctor | |
| ALKA-SELTZER HEARTBURN
anhydrous citric acid and sodium bicarbonate tablet, effervescent |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Revised: 7/2017
Document Id: 551510ea-a429-0c44-e054-00144ff8d46c
Set id: 672106ae-9e27-4173-b7b7-1f5494643673
Version: 2
Effective Time: 20170724
Bayer HealthCare LLC.