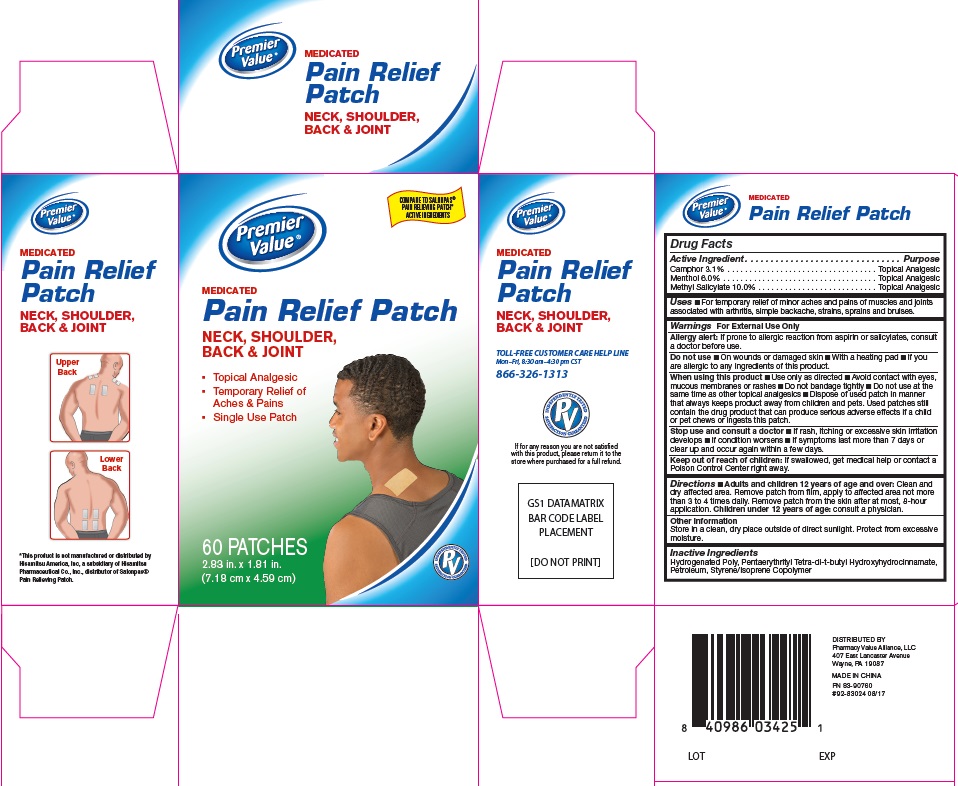
Active Ingredients
Active Ingredients Purpose
Camphor 3.1% ......................Topical Analgesic
Menthol 6.0% .......................Topical Analgesic
Methyl Salicylate 10.0% ........Topical Analgesic
For External Use Only
Allergy alert: If prone to allergic reaction from asprin or salicylates, consult a doctor before use
Do not use:
- On wounds or damaged skin
- With a heating pad
- If you are allergic to any ingredients of this product
When using this product
- Use only as directed
- Avoid contact with eyes, mucous membranes or rashes
- Do not bandage tighly
- Do not use at the same time as other topical analgesics
- Dispose of used patch in manner that keeps product away from childrens and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and consult a doctor
- If rash, itching or excessive skin irritation develops
- If condition worsens
- if symptoms last more than 7 days or clear up and occur again with a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Uses
For temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, strains, sprains, and bruises.
Directions
Adults and children 12 years of age and over: Clean and dry affected area. Remove patch from film, apply to affected area not more than 3 to 4 times daily. Remove patch from the skin after at most, 8-hour application.
Children under 12 years of age: consult physician.