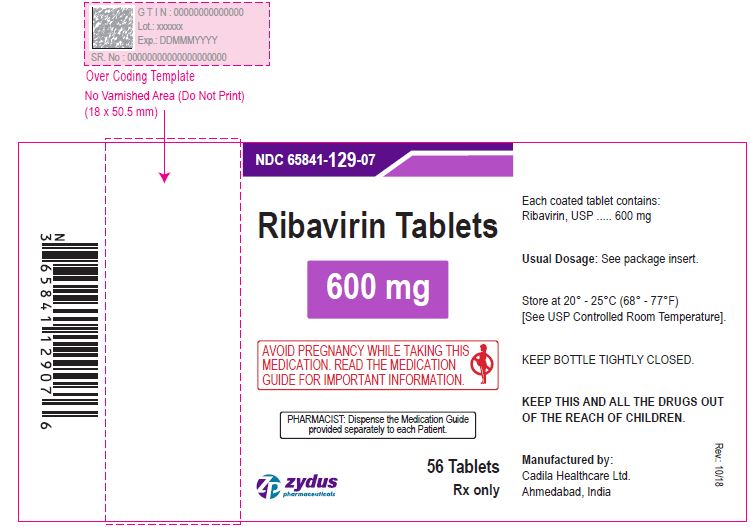
NDC 65841-046-03 in bottle of 168 tablets
Ribavirin Tablets , 200 mg
Rx only
168 tablets
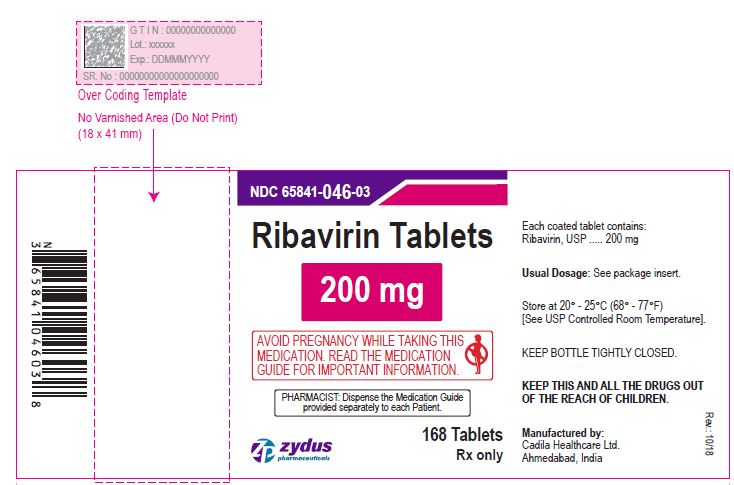
NDC 65841-603-07 in bottle of 56 tablets
Ribavirin Tablets , 400 mg
Rx only
56 tablets
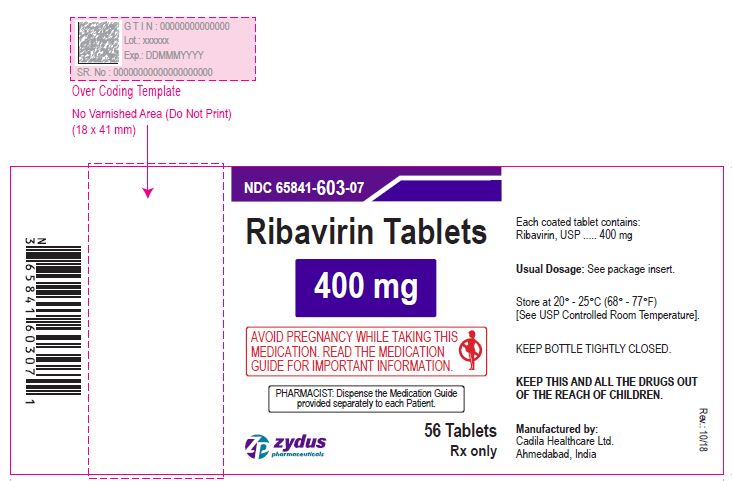
NDC 65841-632-07 in bottle of 56 tablets
Ribavirin Tablets , 500 mg
Rx only
56 tablets
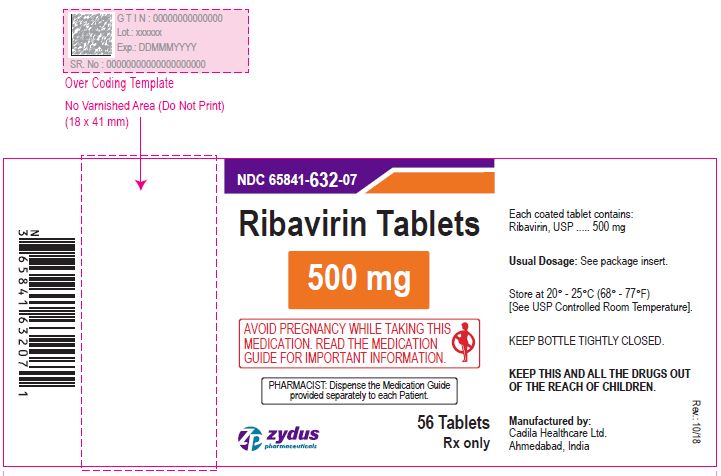
NDC 65841-129-07 in bottle of 56 tablets
Ribavirin Tablets, 600 mg
Rx only
56 tablets