Warnings
Directions
Apply liberally 15 minutes before sun exposure. • Use a water resistant sunscreen if swimming or sweating. • Reapply at least every 2 hours. • Children under 6 months: ask a doctor.
Inactive Ingredients
Water, Propylene Glycol, Glycerin, Dimethicone, VP/Eicosene Copolymer ,Cyclohexasiloxane, Stearic Acid, Potassium Cetyl Phosphate, Dimethiconol, Glyceryl Stearate, PEG-100 Stearate, Aluminum Hydroxide, Disodium EDTA, Tocopherol, Phenoxyethanol, Ethylparaben, Chlorphenesin, Cetyl Alcohol, Acrylates/C10-30 Alkyl Acrylate, Crosspolymer, Methylparaben, Xanthan Gum, Sodium Hydroxide
Principal Display Panel - 1.7oz carton
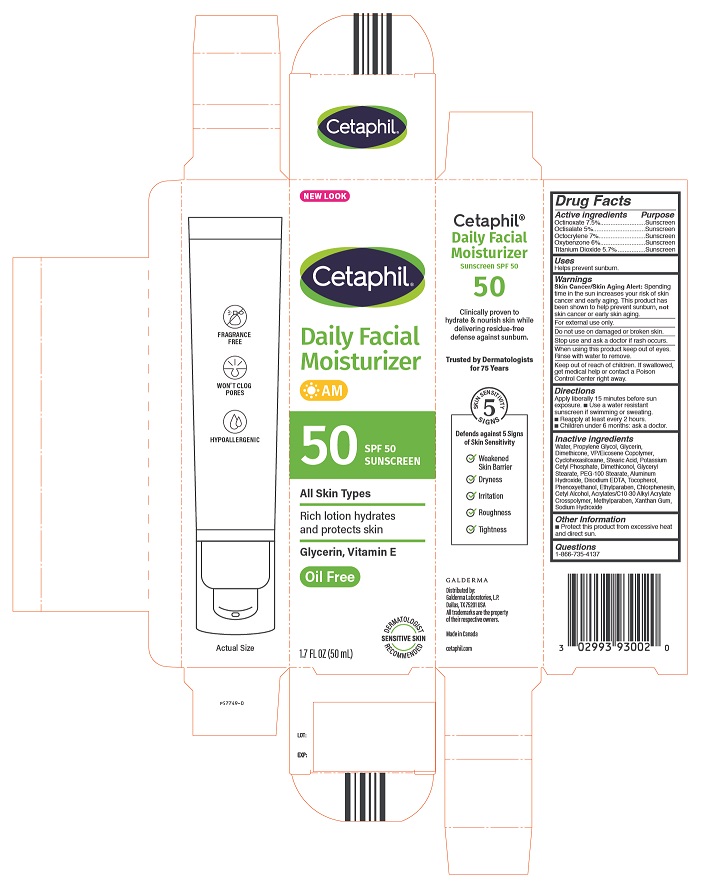
NEW LOOK
Cetaphil®
Daily Facial
Moisturizer
AM
50 SPF 50
Sunscreen
All Skin Types
Rich lotion hydrates
and protects skin
Glycerin, Vitamin E
Oil Free
Dermatologist Recommended
Sensitive Skin
1.7 FL OZ (50 mL)
Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
Made in Canada
cetaphil.com
P57749-0