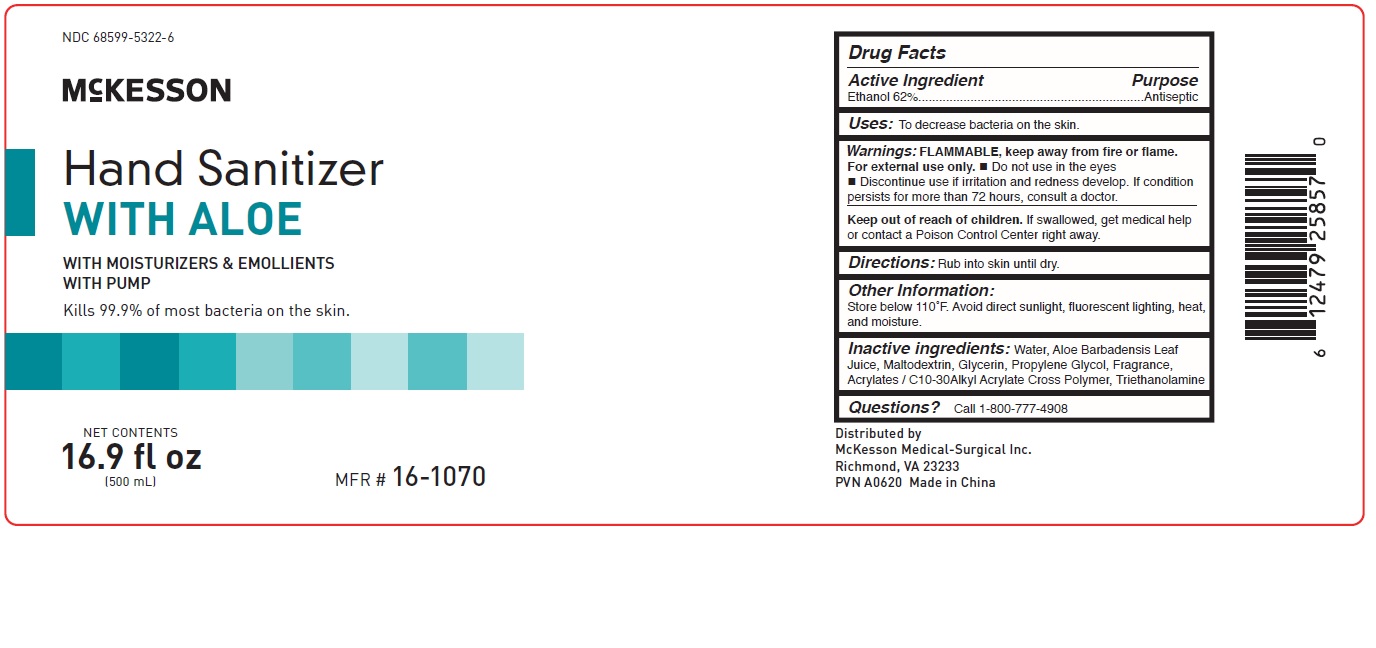
Warnings:
FLAMMABLE, keep away from fire or flame.
For external use only.
- Do not use in the eyes
- Discontinue use if irritation and redness develop. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Other Information:
Store below 110F. Avoid direct sunlight, fluorescent lighting, heat, and moisture.
Inactive Ingredients:
Water, Aloe Barbadensis Leaf Juice, Maltodextrin,
Glycerin, Propylene Glycol, Fragrance,
Acrylates/C10-30 Alkyl Acrylate Cross Polymer,
Triethanolamine
NDC 68599-5322-1
McKesson Hand Sanitizer
With Aloe
With Moisturizers and Emollients
For frequent use
Net Content
4 fl oz (118mL)
MFR# 16-1068

NDC 68599-5322-2
McKesson Hand Sanitizer
With Aloe
With Moisturizers and Emollients
For frequent use
Net Content
4 fl oz (118mL)
24 per case
Warning: Flammable, keep away from fire or flame
Store below 110F (43C)

NDC 68599-5322-4
McKesson Hand Sanitizer
With Aloe
With Moisturizers and Emollients with Pump
For frequent use
Net Content
8 fl oz (237mL)
MFR# 16-1069

NDC 68599-5322-5
McKesson Hand Sanitizer
With Aloe
With Moisturizers and Emollients with Pump
Net Content
8 fl oz (237mL)
24 per Case
MFR# 16-1069
Warning: Flammable, keep away from fire or flame.