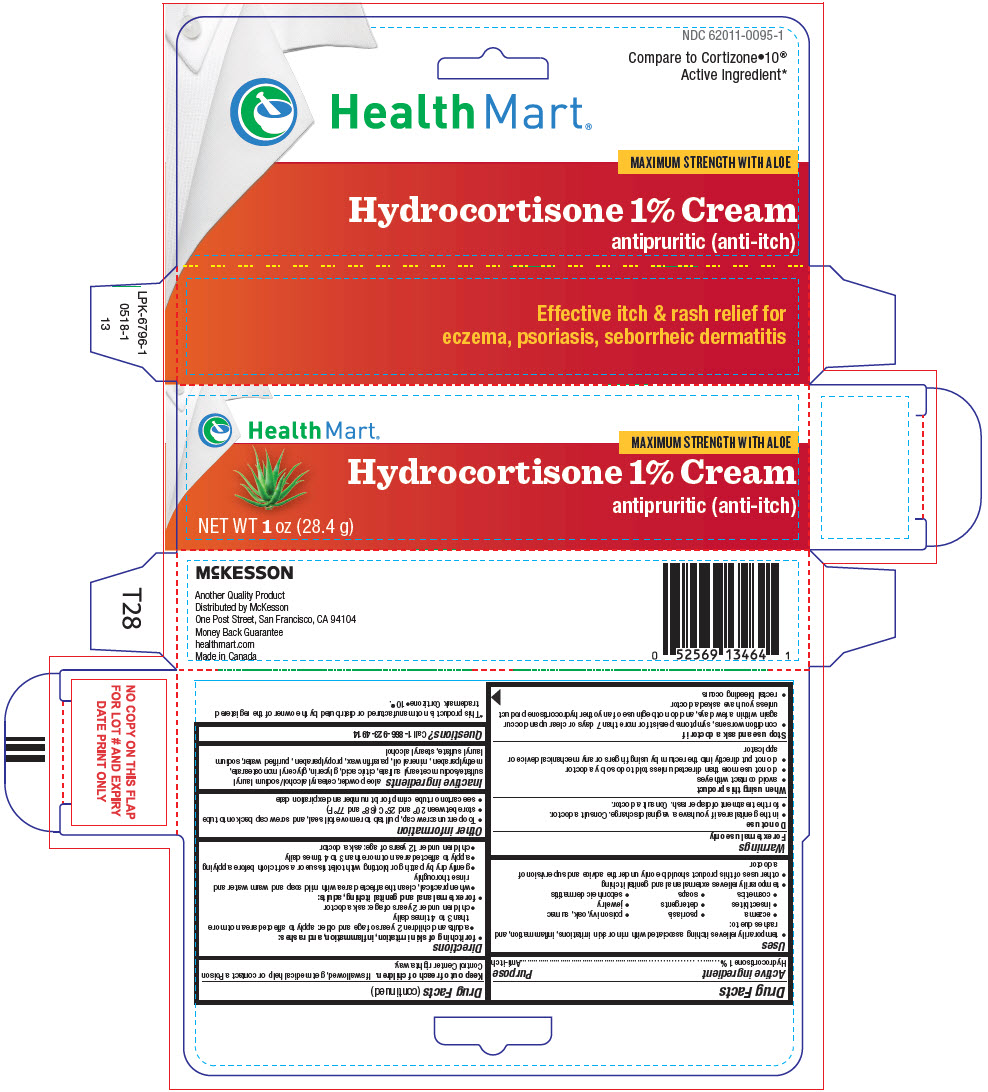
Uses
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- eczema
- psoriasis
- poison ivy, oak, sumac
- insect bites
- detergents
- jewelry
- cosmetics
- soaps
- seborrheic dermatitis
- temporarily relieves external anal and genital itching
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Do not use
- in the genital area if you have a vaginal discharge. Consult a doctor.
- for the treatment of diaper rash. Consult a doctor.
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
- do not put directly into the rectum by using fingers or any mechanical device or applicator
Directions
-
for itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
-
for external anal and genital itching, adults:
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
Other information
- To open: unscrew cap, pull tab to remove foil seal, and screw cap back onto tube
- store between 20° and 25°C (68° and 77°F)
- see carton or tube crimp for lot number and expiration date