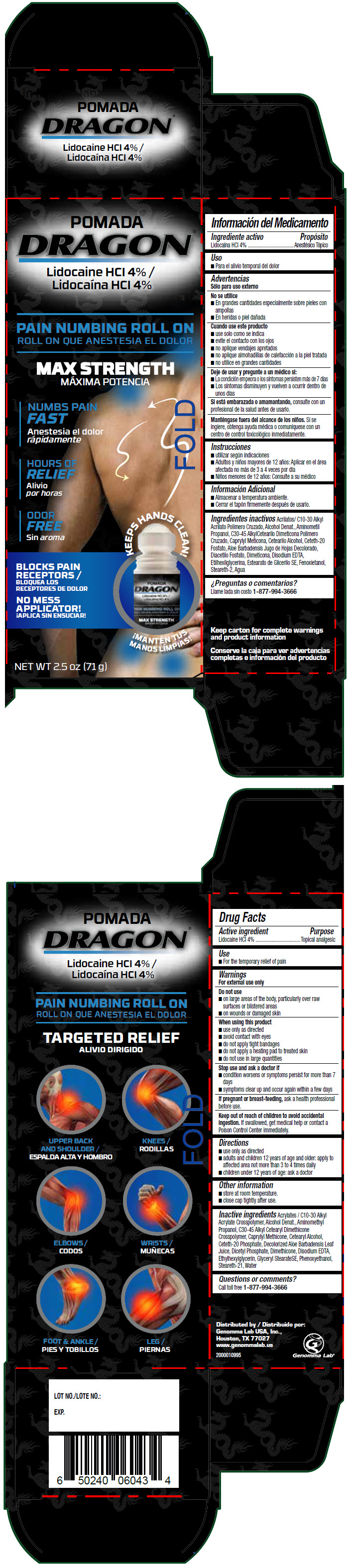
DRAGON PAIN NUMBING ROLL-ON- lidocaine hydrochloride liquid
Genomma Lab USA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Lidocaine HCl 4%
Purpose
Topical analgesic
Use
- For the temporary relief of pain
Warnings
For external use only
Do not use
- on large areas of the body, particularly over raw surfaces or blistered areas
- on wounds or damaged skin
When using this product
- use only as directed
- avoid contact with eyes
- do not apply tight bandages
- do not apply a heating pad to treated skin
- do not use in large quantities
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children to avoid accidental ingestion. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- use only as directed
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
Other information
- store at room temperature.
- close cap tightly after use.
Inactive ingredients
Acrylates / C10-30 Alkyl Acrylate Crosspolymer, Alcohol Denat., Aminomethyl Propanol, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Caprylyl Methicone, Cetearyl Alcohol, Ceteth-20 Phosphate, Decolorized Aloe Barbadensis Leaf Juice, Dicetyl Phosphate, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glyceryl StearateSE, Phenoxyethanol, Steareth-21, Water
Questions or comments?
Call toll free 1-877-994-3666
Distributed by
Genomma Lab USA, Inc.,
Houston, TX 77027
PRINCIPAL DISPLAY PANEL - 71 g Container Carton
POMADA
DRAGON®
Lidocaine HCl 4%
PAIN NUMBING ROLL ON
MAX STRENGTH
NUMBS PAIN
FAST
HOURS OF
RELIEF
ODOR
FREE
KEEPS HANDS CLEAN!
BLOCKS PAIN
RECEPTORS
NO MESS
APPLICATOR!
NET WT 2.5 oz (71 g)