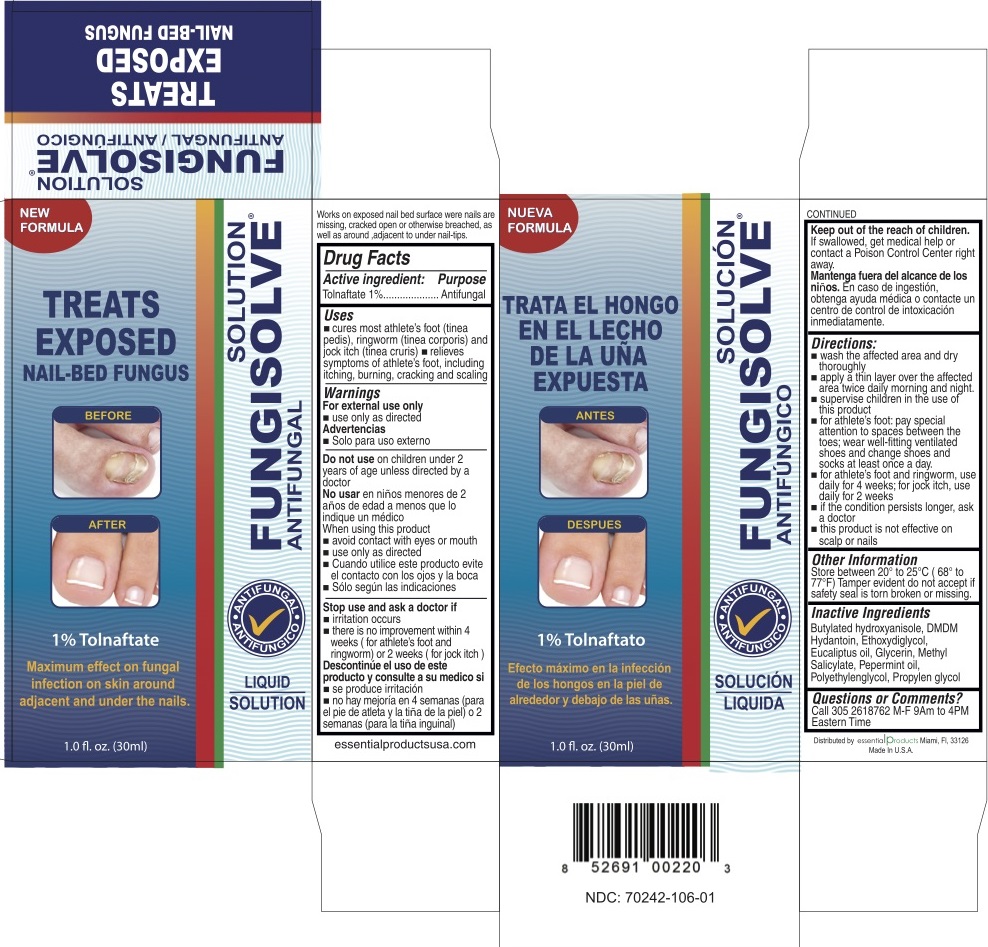
Uses
cures most athlete's foot (tinea pedis), ringworm (tinea corporis) and jock itch (tinea cruris)
relieves sympotoms of athelete's foot including itching, burning, cracking and scaling.
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 4 weeks (for athelete's foot and ringworm) or 2 weeks (for jock itch)
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash the affected area and dry thoroughtly
- apply a thin layer over the affected area twice daily morning and night
- supervice children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes: wear well-fitting ventilated shoes and change shoes and socks at least once a day.
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if the condiotion persists longer, ask a doctor
- this product is not effective on scalp or nails
Other Information
store between 20 to 25 C (68 to 77)F
Tamper evident do not accept if safety seal is broken or missing.