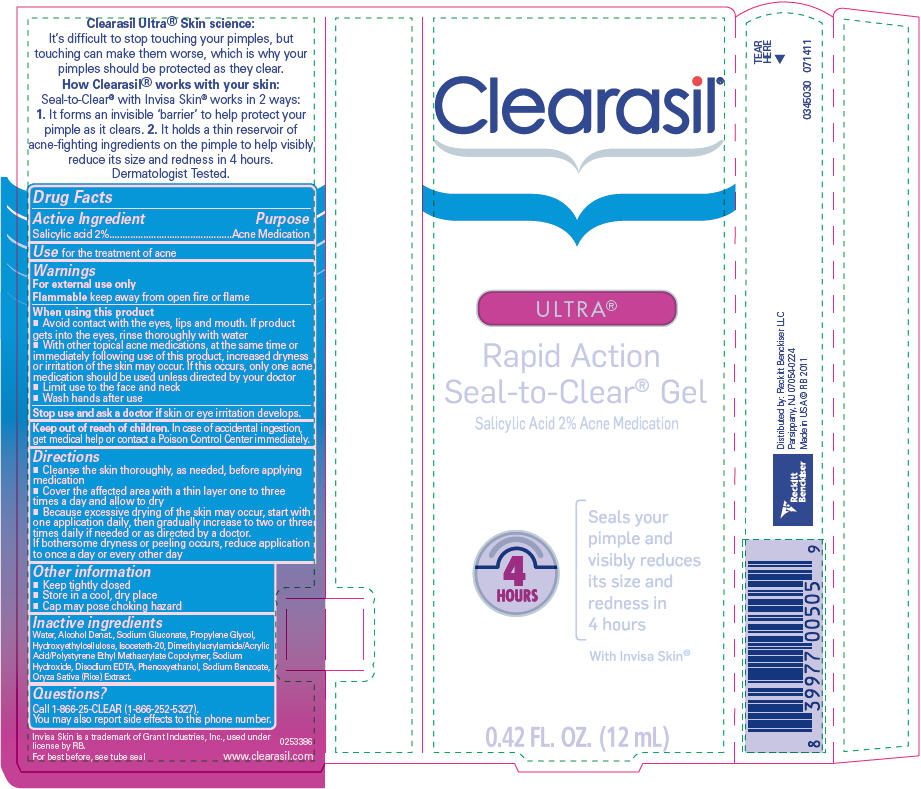
Warnings
For external use only
When using this product
- Avoid contact with the eyes, lips and mouth. If product gets into the eyes, rinse thoroughly with water
- With other topical acne medications, at the same time or immediately following use of this product, increased dryness or irritation of the skin may occur. If this occurs, only one acne medication should be used unless directed by your doctor
- Limit use to the face and neck
- Wash hands after use
Directions
- Cleanse the skin thoroughly, as needed, before applying medication
- Cover the affected area with a thin layer one to three times a day and allow to dry
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
If bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
Water, Alcohol Denat., Sodium Gluconate, Propylene Glycol, Hydroxyethylcellulose, Isoceteth-20, Dimethylacrylamide/Acrylic Acid/Polystyrene Ethyl Methacrylate Copolymer, Sodium Hydroxide, Disodium EDTA, Phenoxyethanol, Sodium Benzoate, Oryza Sativa (Rice) Extract.