50 mg/mL
For intramuscular use in swine.
Not for use in breeding swine.
PRODUCT
INFORMATION
Approved by FDA under NADA # 101-479
DESCRIPTION
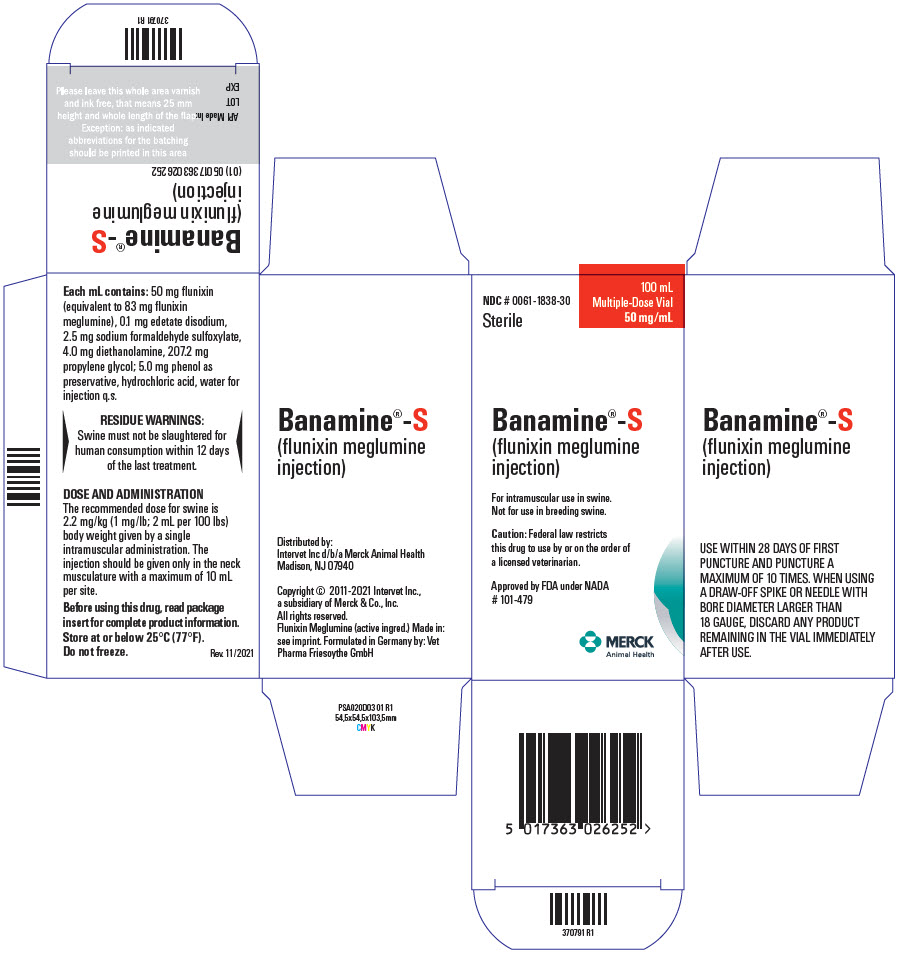
Each milliliter of BANAMINE-S (flunixin meglumine injection) contains 50 mg flunixin (equivalent to 83 mg flunixin meglumine), 0.1 mg edetate disodium, 2.5 mg sodium formaldehyde sulfoxylate, 4.0 mg diethanolamine, 207.2 mg propylene glycol; 5.0 mg phenol as preservative, hydrochloric acid, water for injection q.s.
CLINICAL PHARMACOLOGY
Flunixin meglumine is a potent non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test.
Flunixin is known to persist in inflammatory tissues1 and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentrations2. Therefore, prediction of drug concentrations based upon estimated plasma terminal elimination half-life will likely underestimate both the duration of drug action and the concentration of drug remaining at the site of activity.
The pharmacokinetic profiles were found to follow a 2-compartmental model, although a deep (third) compartment was observed in some animals. The mean terminal elimination half-life (β half-life) of flunixin after a single intramuscular injection of Banamine (2.2 mg/kg) to pigs was between 3 and 4 hours. The mean observed maximum plasma concentration was 2944 ng/mL, achieved at a mean time of approximately 0.4 hours. The mean AUC(0-LOQ) was 6431 ng*hr/mL. Following IM administration of flunixin, quantifiable drug concentration could be measured up to 18 hours post dose. The mean volume of distribution was 2003 mL/kg and the mean total clearance was 390 mL/hr/kg. The mean absolute bioavailability of flunixin following an intramuscular injection in the neck was 87%.
INDICATION
BANAMINE-S (flunixin meglumine injection) is indicated for the control of pyrexia associated with swine respiratory disease.
DOSE AND ADMINISTRATION
The recommended dose for swine is 2.2 mg/kg (1 mg/lb; 2 mL per 100 lbs) body weight given by a single intramuscular administration. The injection should be given only in the neck musculature with a maximum of 10 mL per site.
USE WITHIN 28 DAYS OF FIRST PUNCTURE AND PUNCTURE A MAXIMUM OF 10 TIMES. WHEN USING A DRAW-OFF SPIKE OR NEEDLE WITH BORE DIAMETER LARGER THAN 18 GAUGE, DISCARD ANY PRODUCT REMAINING IN THE VIAL IMMEDIATELY AFTER USE.
Note: Intramuscular injection may cause local tissue irritation and damage. In an injection-site irritation study, the tissue damage did not resolve in all animals by Day 28 post-injection. This may result in trim loss of edible tissue at slaughter.
CONTRAINDICATIONS
There are no known contraindications to this drug in swine when used as directed. Do not use in animals showing hypersensitivity to flunixin meglumine. Use judiciously when renal impairment or gastric ulceration is suspected.
RESIDUE WARNINGS
Swine must not be slaughtered for human consumption within 12 days of the last treatment.
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed.
Since many NSAIDs possess the potential to produce gastrointestinal ulceration, concomitant use of flunixin meglumine with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided.
Not for use in breeding swine. The reproductive effects of BANAMINE-S (flunixin meglumine injection) have not been investigated in this class of swine.
Intramuscular injection may cause local tissue irritation and damage. In an injection site irritation study, the tissue damage did not resolve in all animals by Day 28 post-injection. This may result in trim loss of edible tissue at slaughter.
ADVERSE REACTIONS
Flunixin was mildly irritating at the injection sites. No other flunixin-related changes (adverse reactions) were noted in swine administered a 1× (2.2 mg/kg; 1.0 mg/lb) dose for 9 days.
ANIMAL SAFETY
Minimal toxicity manifested itself as statistically significant increased spleen weight at elevated doses (5× or higher daily for 9 days) with no change in normal microscopic architecture.
HOW SUPPLIED
BANAMINE-S (flunixin meglumine injection), 50 mg/mL is available in 100-mL (NDC # 0061-1838-30) multi-dose vials.
1. Lees P, Higgins AJ. Flunixin inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984; 37:347-349.
2. Odensvik K. Pharmacokinetics of flunixin and its effect on prostaglandin F2α metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacol Ther. 1995; 18:254-259.
Distributed by:
Intervet Inc d/b/a Merck Animal Health
Madison, NJ 07940
Copyright © 2011-2021 Intervet Inc., a subsidiary of Merck & Co., Inc.
All rights reserved.
Formulated in Germany by: Vet Pharma Friesoythe GmbH
Rev. 11/2021
368569 R1
PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
NDC # 0061-1838-30
Sterile
100 mL
Multiple-Dose Vial
50 mg/mL
Banamine®-S
(flunixin meglumine
injection)
For intramuscular use in swine.
Not for use in breeding swine.
Caution: Federal law restricts
this drug to use by or on the order of
a licensed veterinarian.
Approved by FDA under NADA
# 101-479
MERCK
Animal Health