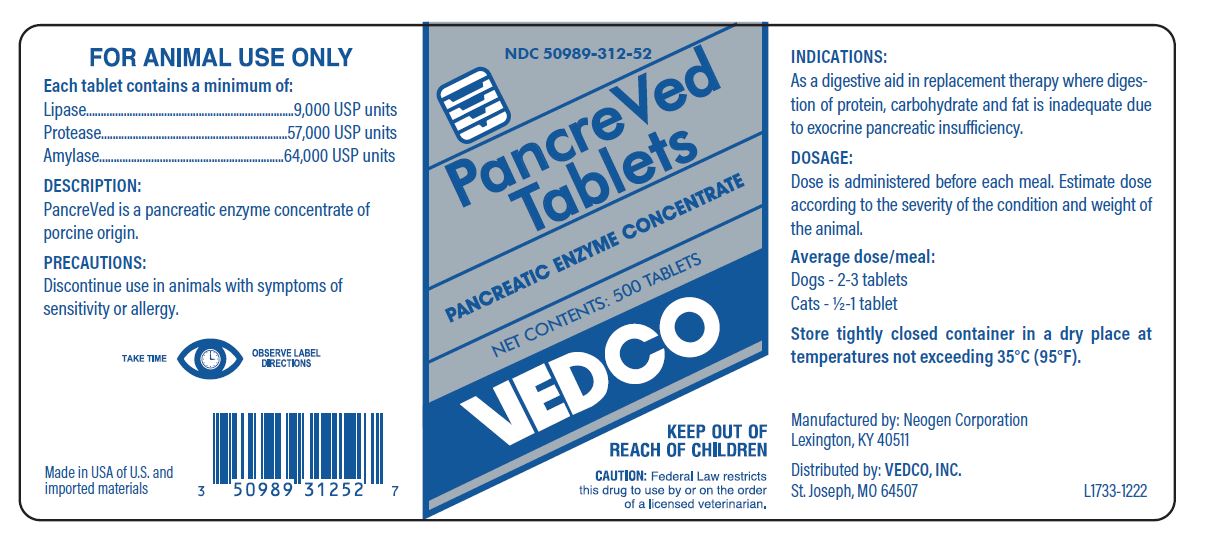
PancreVed Tablets
PANCREATIC ENZYME CONCENTRATE
VEDCO
KEEP OUT OF REACH OF CHILDREN
CAUTION: Federal Law restricts this drug to use by or on the order of a licensed veterinarian.
Each tablet contains a minimum of:
Lipase.........................9,000 USP units
Protease...................57,000 USP units
Amylase....................64,000 USP units
DESCRIPTION:
PancreVed is a pancreatic enzyme concentrate of porcine origin.
TAKE TIME
OBSERVE LABEL DIRECTIONS
Manufactured by Neogen Corporation
Lexington, KY 40511
Distributed by:
VEDCO, INC
St. Joseph, MO 64507
Made in USA of U.S. and imported materials
DOSAGE:
Dose is administered before each meal. Estimate dose according to the severity of the condition and weight of the animal.
Average dose/meal:
Dogs - 2 - 3 tablets
Cats - ½ - 1 tablet
INDICATIONS:
As a digestive aid in replacement therapy where digestion of protein, carbohydrate and fat is inadequate due to exocrine pancreatic insufficiency.