Uses
- temporarily relieves
- sneezing
- itchy nose or throat
- runny nose
- itchy, watery eyes due to hay fever
- nasal and sinus congestion
- cough due to minor throat and bronchial irritation as may occur with a cold
Warnings
Do not use
- in a child under 6 years of age
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if the child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- with any other product containing diphenhydramine, even one used on skin
- for the purpose of making your child sleepy
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts, or as occurs with asthma
- a breathing problem such as chronic bronchitis
When using this product
- do not exceed recommended dosage
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- excitability may occur, especially in children
Directions
- may be given every 4 hours. Do not give more than 6 doses in 24 hours unless directed by a doctor.
- to find right dose, use rotating bottle label to dose by weight; otherwise, use chart below to dose by age.
- specifically designed for use with enclosed dosing spoon. Use only enclosed dosing spoon to dose this product. Do not use any other dosing device.
| children under 6 years of age | do not use |
| children 6 to under 12 years of age | 1-2 tsp. (5-10 mL) |
Other information
- each teaspoonful contains: sodium 4 mg
- store at controlled room temperature 20°-25°C (68°-77°F)
Inactive ingredients
artificial flavor, benzoic acid, citric acid, disodium edetate, FD&C blue no. 1, FD&C red no. 40, propylene glycol, purified water, sodium citrate, sodium saccharin, sorbitol.
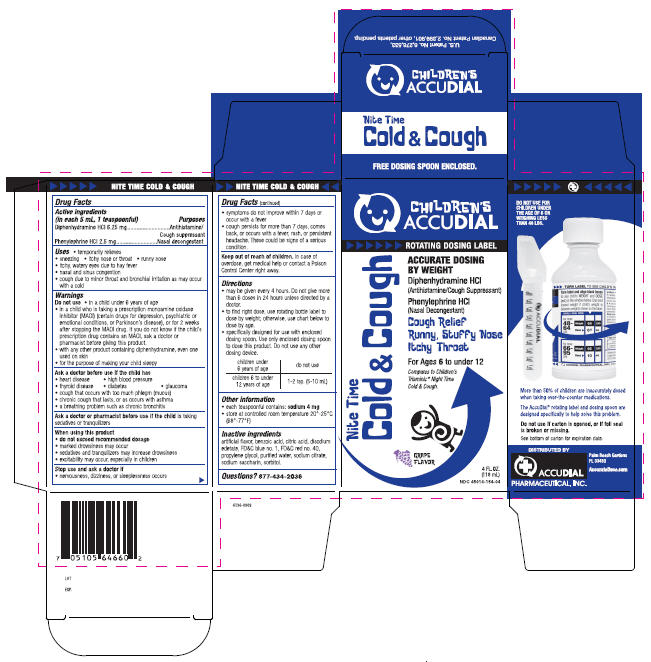
PRINCIPAL DISPLAY PANEL - 118 mL Carton
CHILDREN'S
ACCUDIAL
ROTATING DOSING LABEL
Nite Time
Cold & Cough
ACCURATE DOSING
BY WEIGHT
Diphenhydramine HCl
(Antihistamine/Cough Suppressant
Phenylephrine HCl
(Nasal Decongestant)
Cough Relief
Runny, Stuffy Nose
Itchy Throat
For Ages 6 to under 12
Compares to Children's
Triaminic® Night Time
Cold & Cough.
GRAPE
FLAVOR
4 FL. OZ.
(118 mL)
NDC 45014-154-04