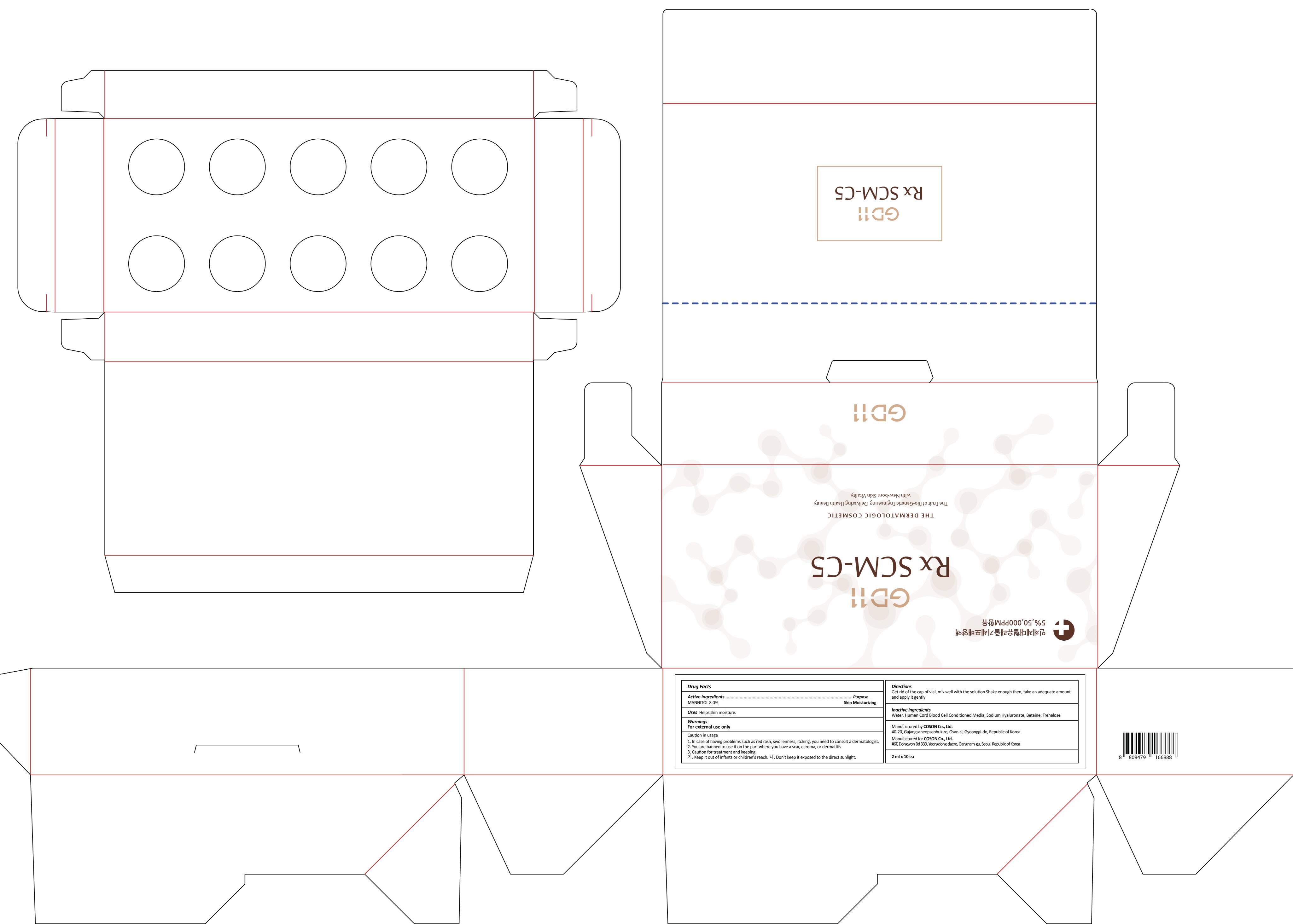
INACTIVE INGREDIENT
Inactive ingredients: Water, Human Cord Blood Cell Conditioned Media, Sodium Hyaluronate, Betaine, Trehalose
WARNINGS
Warnings: For external use only
Caution in usage 1. In case of having problems such as red rash, swollenness, itching, you need to consult a dermatologist. 2. You are banned to use it on the part where you have a scar, eczema, or dermatitis 3. Caution for treatment and keeping. - Keep it out of infants or children's reach. - Don't keep it exposed to the direct sunlight