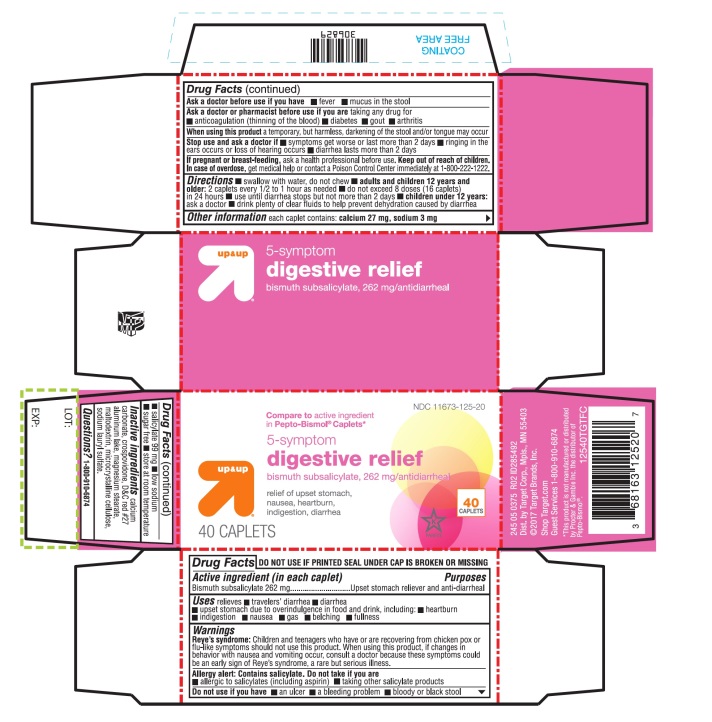
Warnings
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert:
Contains salicylate. Do not take if you are
- ▪
- allergic to salicylates (including aspirin)
- ▪
- taking other salicylate products
Ask a doctor before use if you are taking any drug for
- ▪
- anticoagulation (thinning of the blood)
- ▪
- diabetes
- ▪
- gout
- ▪
- arthritis
Directions
- ▪
- swallow with water, do not chew
- ▪
- adults and children 12 years and older: 2 caplets every 1/2 to 1 hour as needed
- ▪
- do not exceed 8 doses (16 caplets) in 24 hours
- ▪
- use until diarrhea stops but not more than 2 days
- ▪
- children under 12 years: ask a doctor
- ▪
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
Other information
- ▪
- each caplet contains: calcium 27 mg
- ▪
- sodium 3 mg
- ▪
- salicylate 99 mg
- ▪
- low sodium
- ▪
- sugar free
- ▪
- avoid excessive heat (over 104°F or 40°C)
Inactive ingredients
calcium carbonate, crospovidone, D&C red#27 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate.
PRINCIPAL DISPLAY PANEL -
NDC 11673-125-20
Compare to active ingredient in Pepto-Bismol® Caplets*
5- symptom digestive relief
relief of upset stomach, nausea, heartburn, indigestion, diarrhea
40 CAPLETS
*This product is not manufactured or distributed by Procter & Gamble Inc. the distributor of PEPTO-BISMOL®
TAMPER EVIDENT DO NOT USE IF PRINTED SEAL UNDER CAP IS TORN OR MISSING.
245 05 0375 R01 ID285492
Dist.by Target Corp., Mpls ., MN 55403
©2017 Target Brands, Inc.
Shop Target.com
Guest Services 1-800-910-6874