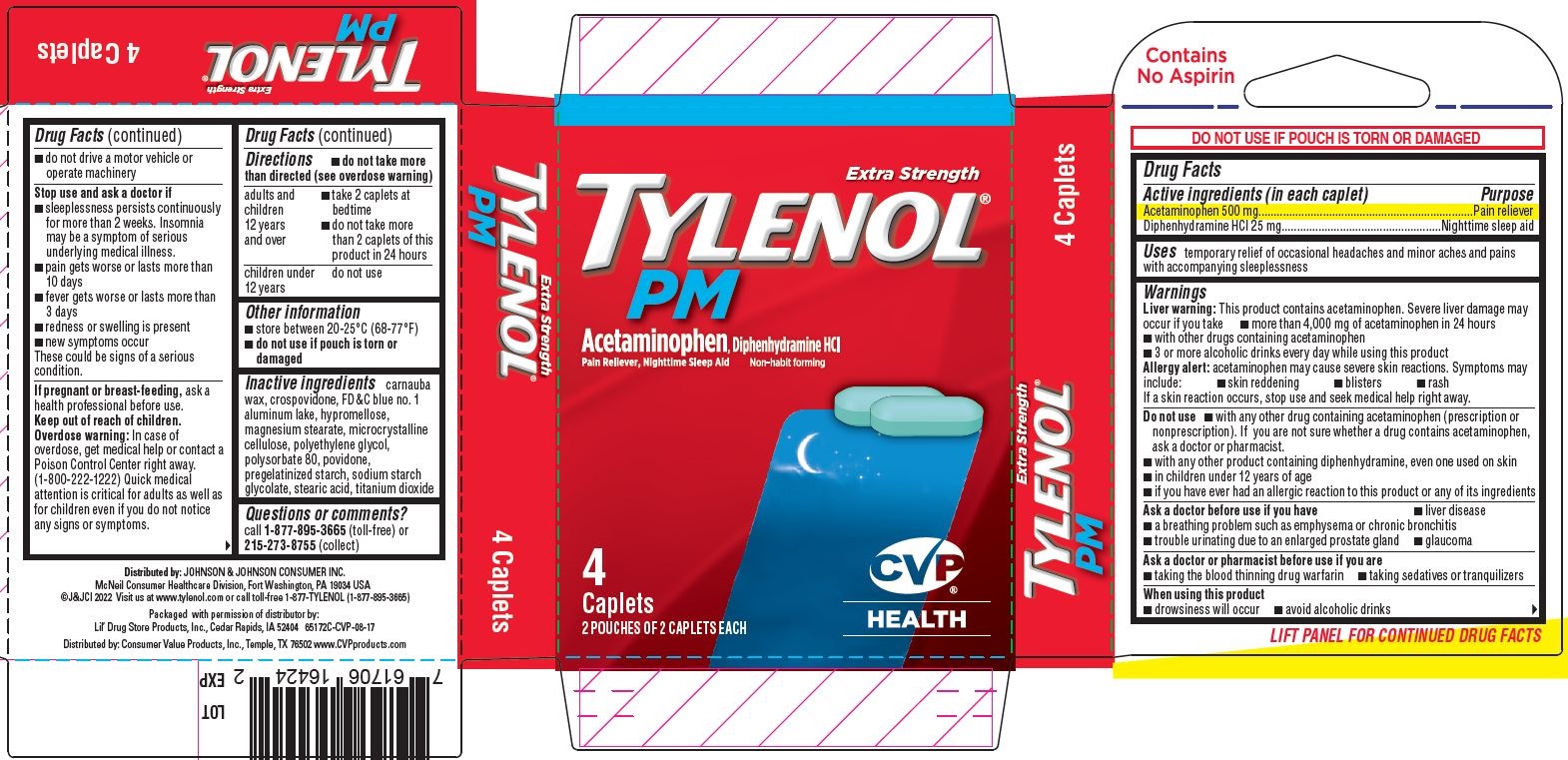
Uses
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years | do not use |
Inactive ingredients
carnauba wax, crospovidone, FD&C blue no. 1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, stearic acid, titanium dioxide
Product distributed by: JOHNSON & JOHNSON CONSUMER INC.
McNeil Consumer Healthcare Division, Fort Washington, PA 19034 USA
Repackaged and distributed by: Lil' Drug Store Products, Inc., 9300 Earhart Lane SW
Cedar Rapids, IA 52402