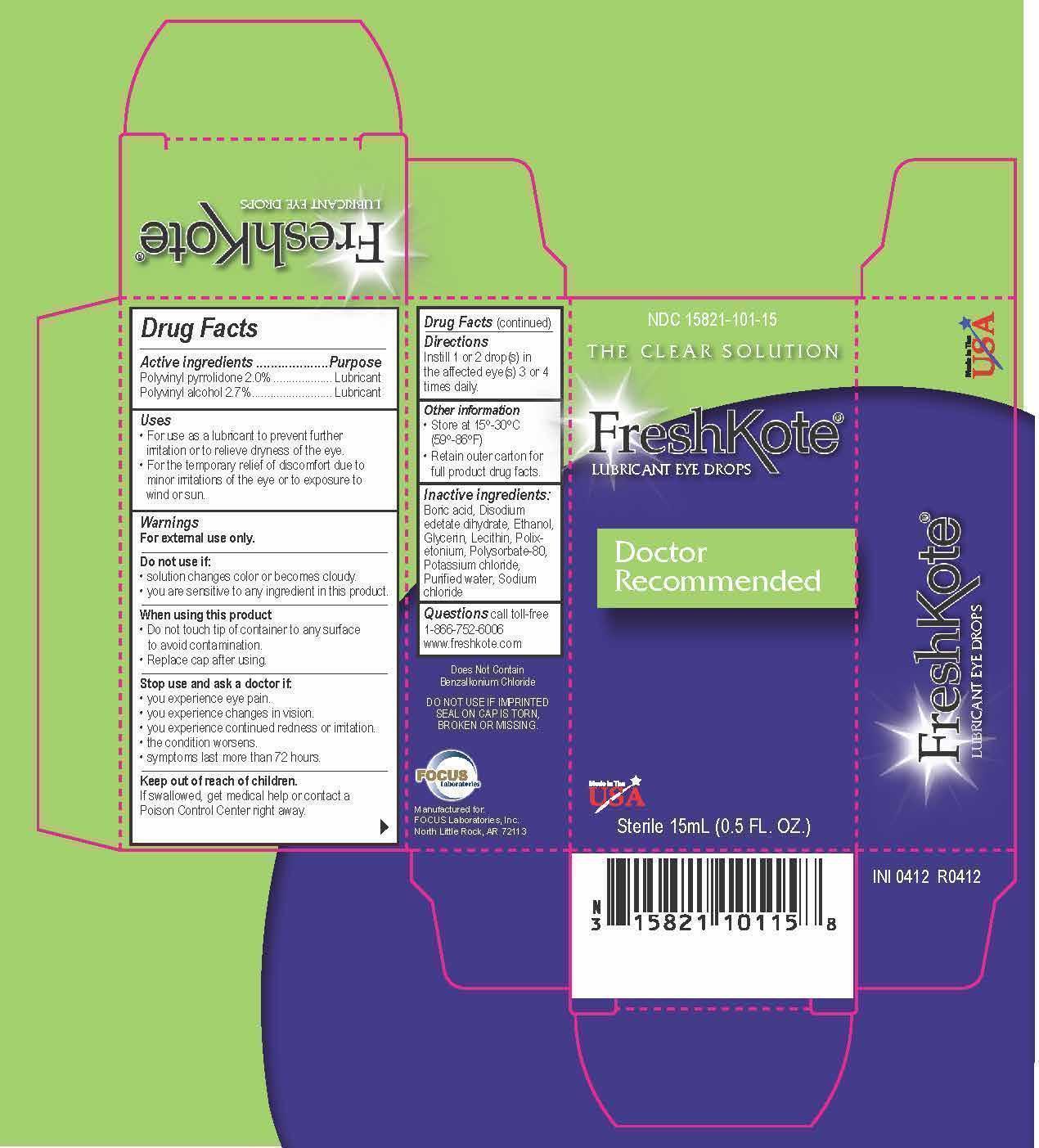
Active ingredients
Active ingredients......................Purpose
Polyvinyl pyrrolidone2.0%..............Lubricant
Polyvinyl alcohol 2.7%....................Lubricant
Uses
- For use as a lubricant to prevent further irritation or to relieve dryness of the eye.
- For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
Warnings
For external use only.
Do not use if:
- solution changes color or becomes cloudy.
- you are sensitive to any ingredient in this product.
When using this product:
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after using.
Stop use and ask a doctor if:
- you experience eye pain.
- you experience changes in vision.
- you experience continued redness or irritation.
- the condition worsens.
- symptoms last more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients:
Boric acid, Disodium edetate dihydrate, Ethanol, Glycerin, Lecithin, Polixetonium, Polysorbate-80, Potassium chloride, Purified water, Sodium chloride