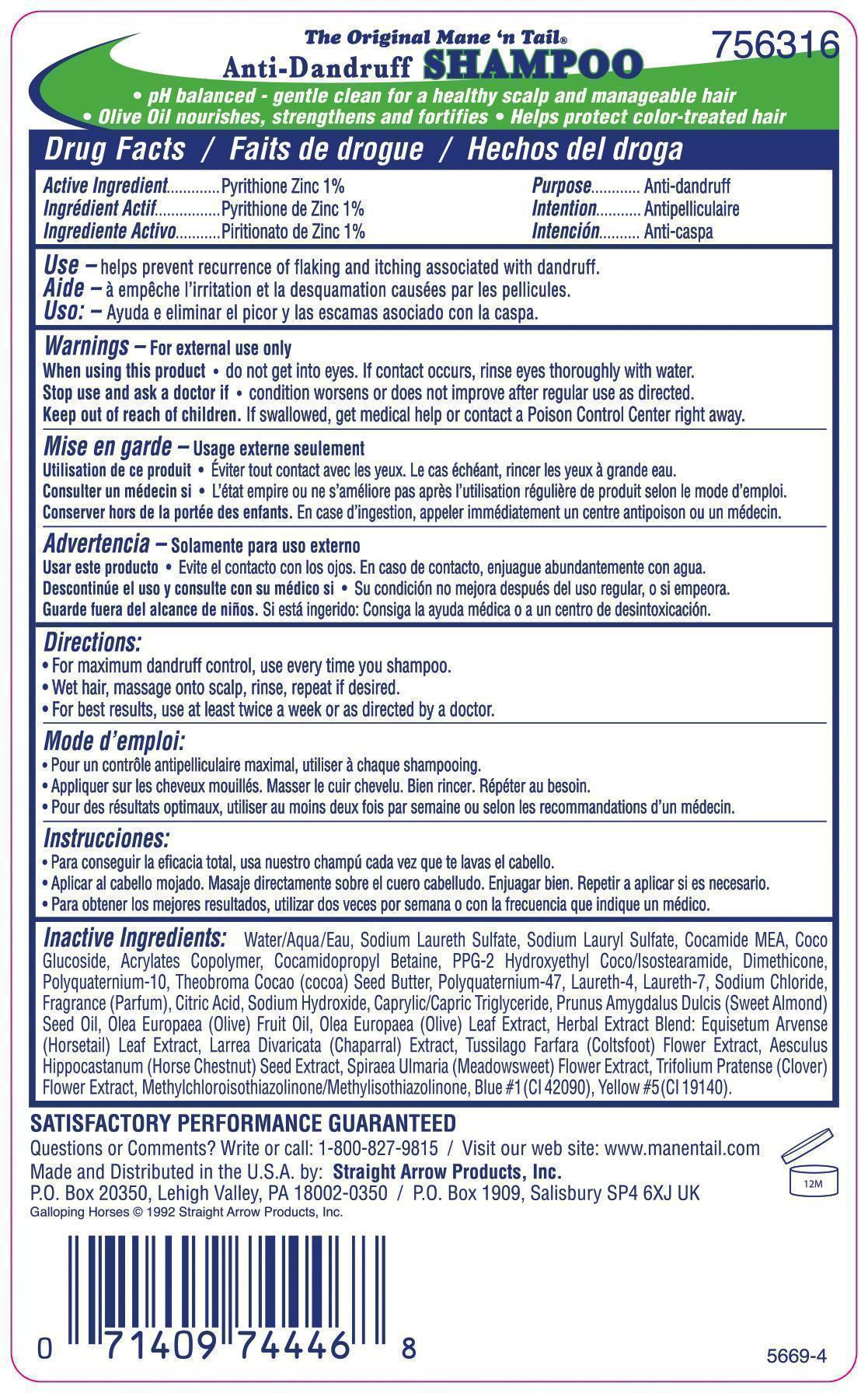
Directions:
- For maximum dandruff control, use every time you shampoo.
- Wet hair, massage onto scalp, rinse, repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive Ingredients: Aqua (Water), Sodium Laureth Sulfate, Sodium Lauryl Sulfate, Cocamide MEA, Coco Glucoside, Acrylates Copolymer, Cocamidopropyl Betaine, PPG-2 Hydroxyethyl Coco/Isostearamide, Dimethicone, Polyquaternium-10, Theobroma Cocao (cocoa) Seed Butter, Polyquaternium-47, Laureth-4, Laureth-7, Sodium Chloride, Fragrance (Parfum), Citric Acid, Sodium Hydroxide, Caprylic/Capric Triglyceride, Prunus Amygdalus Dulcis (Sweet Almond) Seed Oil, Olea Europaea (Olive) Fruit Oil, Olea Europaea (Olive) Leaf Extract, Herbal Extract Blend: Equisetum Arvense (Horsetail) Leaf Extract, Larrea Divaricata (Chaparral) Extract, Tussilago Farfara (Coltsfoot) Flower Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Spiraea Ulmaria (Meadowsweet) Flower Extract, Trifolium Pratense (Clover) Flower Extract, Methylchloroisothiazolinone/ Methylisothiazolinone, Blue #1 (CI 42090), Yellow #5 (CI 19140).