When using this product
- avoid contact with eyes
- if product gets into eyes, rinse thoroughly with water
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
- store at 20°C to 25°C (68°F-77°F)
- see top panel for lot number and expiration date
Inactive ingredients
benzyl alcohol, BHT, blue 1, citric acid, cocamide MEA, fragrance, glycol distearate, hydrochloric acid, hydroxypropyl methylcellulose, polyquaternium-7, sodium chloride, sodium cocoyl sarcosinate, sodium hydroxide, sodium laureth sulfate, tetrasodium EDTA, water
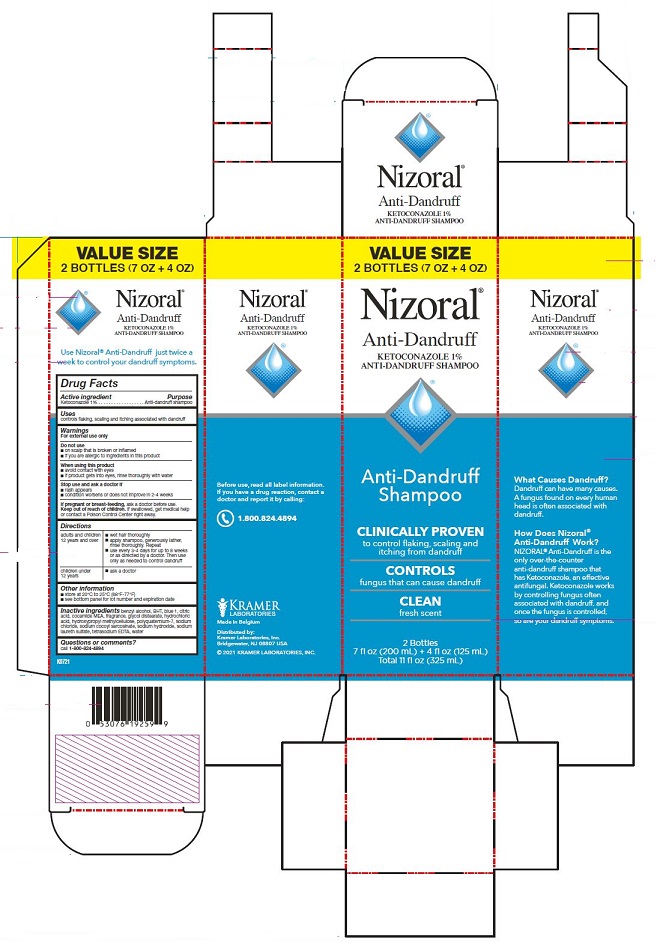
PRINCIPAL DISPLAY PANEL
Nizoral®
Anti-Dandruff
KETOCONAZOLE 1%
ANTI-DANDRUFF SHAMPOO
Anti-Dandruff
Shampoo
CLINICALLY PROVEN
to control flaking, scaling and
itching from dandruff
CONTROLS
fungus that can cause dandruff
CLEAN
fresh scent
7 fl oz (200mL)
What Causes
Dandruff?
Dandruff can have
many causes. A fungus
found on every human
head is often associated
with dandruff.
How Does Nizoral®
Anti-Dandruff
Work?
NIZORAL® is the
only over-the-counter
anti-dandruff shampoo
that has Ketoconazole,
an effective antifungal.
Ketoconazole works
by controlling fungus
often associated with
dandruff, and once the
fungus is controlled,
so are your dandruff
symptoms.
Before use, read all label
information. If you have a
drug reaction, contact a
doctor and report it by
calling:
1.800.824.4894
KRAMER
LABORATORIES
Made in Belgium
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807
USA
© 2022 KRAMER
LABORATORIES, INC.
USA
753769
875
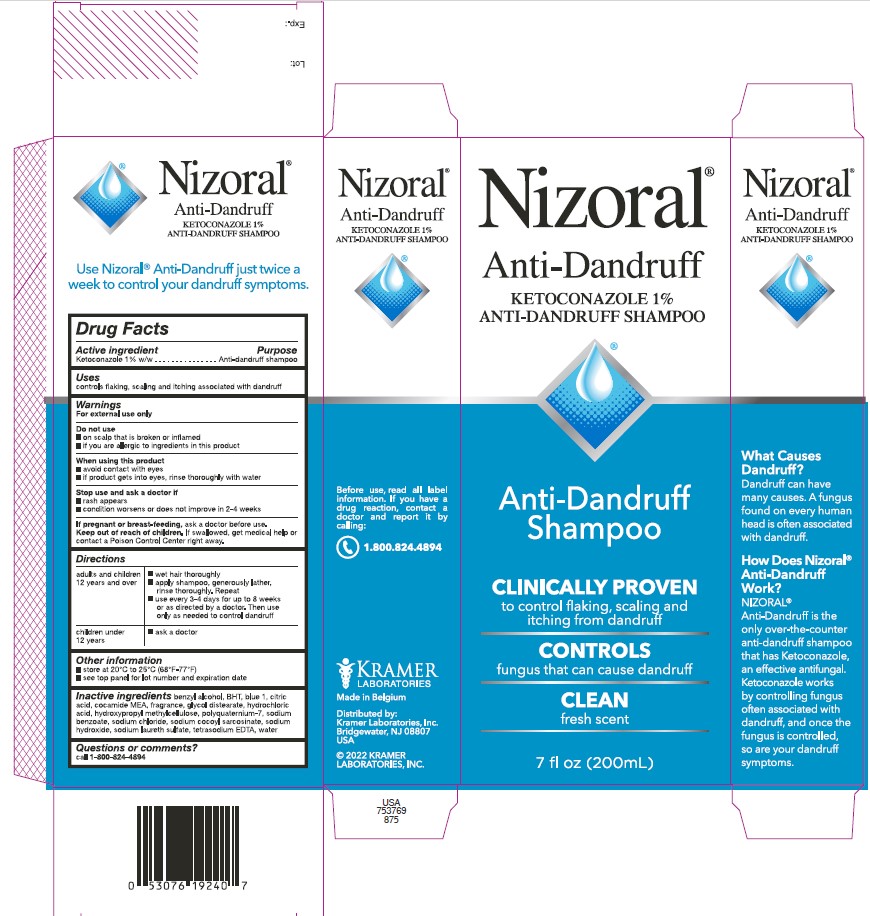
Nizoral®
Anti-Dandruff
KETOCONAZOLE 1%
ANTI-DANDRUFF SHAMPOO
Anti-Dandruff
Shampoo
CLINICALLY PROVEN
to control flaking, scaling and
itching from dandruff
CONTROLS
fungus that can cause dandruff
CLEAN
fresh scent
7 fl oz (200mL)
USA -753767

Dist. by: Kramer Laboratories, Inc., Bridgewater, NJ 08807 USA
© 2022 Kramer Laboratories, Inc.
Made in Belgium
USA - 753768
LOT
EXP

Nizoral®
Anti-Dandruff
KETOCONAZOLE 1%
ANTI-DANDRUFF SHAMPOO
Anti-Dandruff
Shampoo
CLINICALLY PROVEN
to control flaking, scaling and
itching from dandruff
CONTROLS
fungus that can cause dandruff
CLEAN
fresh scent
4 fl oz (125mL)
What Causes
Dandruff?
Dandruff can have
many causes. A
fungus found on
every human head
is often associated
with dandruff.
How Does Nizoral®
Anti-Dandruff
Work?
NIZORAL® is the
only over-the-counter
anti-dandruff
shampoo that has
Ketoconazole, an
effective antifungal.
Ketoconazole works
by controlling fungus
often associated
with dandruff, and
once the fungus is
controlled, so are your
dandruff symptoms.
Before use, read all label
information. If you have
a drug reaction, contact
a doctor and report it by
calling:
1.800.824.4894
KRAMER
LABORATORIES
Made in Belgium
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807
USA
© 2022 KRAMER
LABORATORIES, INC.
USA
753764
876
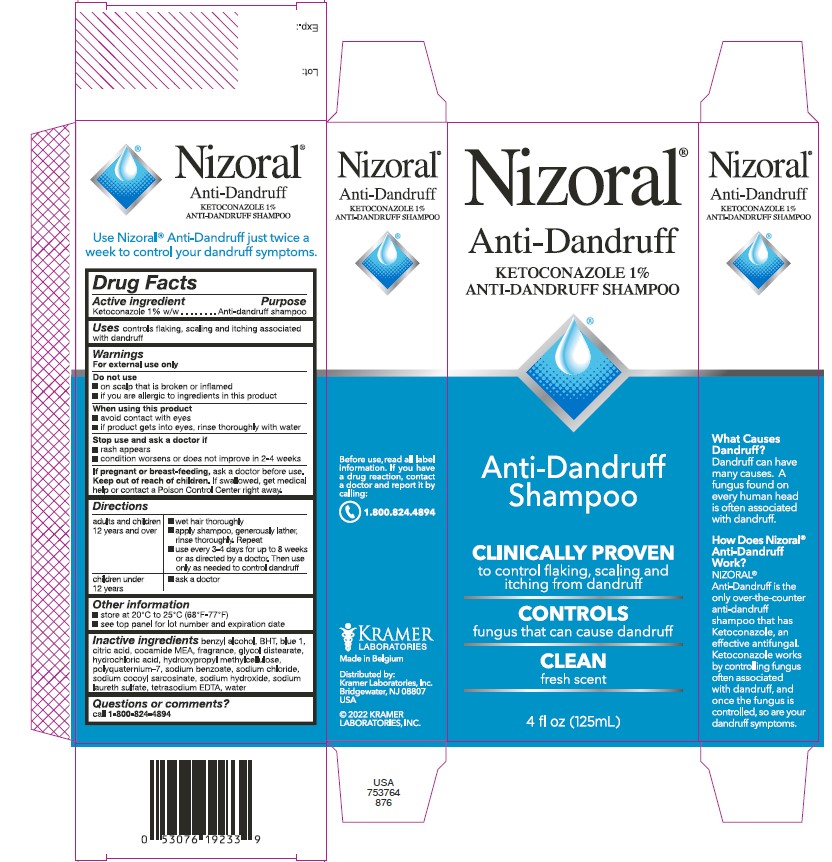
Nizoral®
Anti-Dandruff
KETOCONAZOLE 1%
ANTI-DANDRUFF SHAMPOO
Anti-Dandruff
Shampoo
CLINICALLY PROVEN
to control flaking, scaling and
itching from dandruff
CONTROLS
fungus that can cause dandruff
CLEAN
fresh scent
4 fl oz (125mL)
USA -753765

Dist. by: Kramer Laboratories, Inc. Bridgewater, NJ 08807 USA
© 2022 Kramer Laboratories, Inc.
Made in Belgium
USA - 753766
LOT
EXP

BUY 2 AND SAVE!
Nizoral®
Anti-Dandruff
KETOCONAZOLE 1%
ANTI-DANDRUFF SHAMPOO
Anti-Dandruff
Shampoo
CLINICALLY PROVEN
to control flaking, scaling and
itching from dandruff
CONTROLS
fungus that can cause dandruff
CLEAN
fresh scent
2 Bottles: 7 fl oz (200 mL) each
Total 14 fl oz (400 mL)
Use Nizoral® Anti-Dandruff just
twice a week to control your
dandruff symptoms.
What Causes Dandruff?
Dandruff can have many causes.
A fungus found on every human
head is often associated with
dandruff.
How Does Nizoral®
Anti-Dandruff Work?
NIZORAL® Anti-Dandruff is the
only over-the-counter
anti-dandruff shampoo that
has Ketoconazole, an effective
antifungal. Ketoconazole works
by controlling fungus often
associated with dandruff, and
once the fungus is controlled,
so are your dandruff symptoms.
Before use, read all label
information. If you have a
drug reaction, contact a
doctor and report it by
calling:
1.800.824.4894
KRAMER
LABORATORIES
Made in Belgium
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807 USA
© 2021 KRAMER
LABORATORIES, INC.

value packs xxx