PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
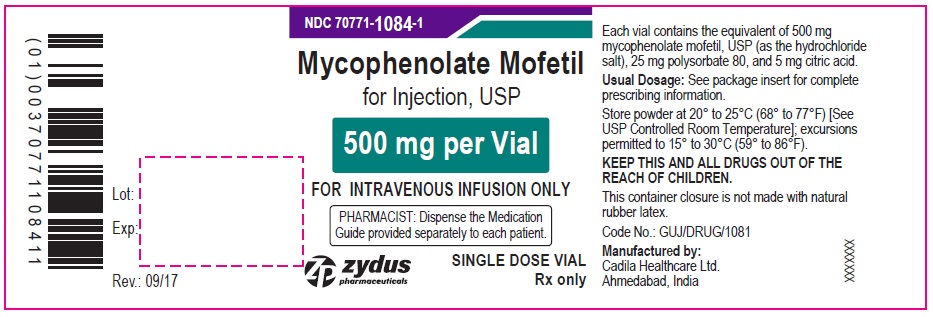
PRINCIPAL DISPLAY PANEL – MYCOPHENOLATE MOFETIL 500 MG CONTAINER LABEL
NDC 70771-1084-1
Mycophenolate Mofetil for Injection, USP
(500 mg per Vial)
FOR INTRAVENOUS INFUSION ONLY
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
SINGLE DOSE VIAL
Rx only
Zydus Pharmaceuticals
PRINCIPAL DISPLAY PANEL - MYCOPHENOLATE MOFETIL 500 MG CARTON LABEL
NDC 70771-1084-8
Mycophenolate Mofetil for Injection, USP
(500 mg per Vial)
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
FOR INTRAVENOUS INFUSION ONLY
SINGLE DOSE VIAL
4 Vials
Rx only
Zydus Pharmaceuticals
