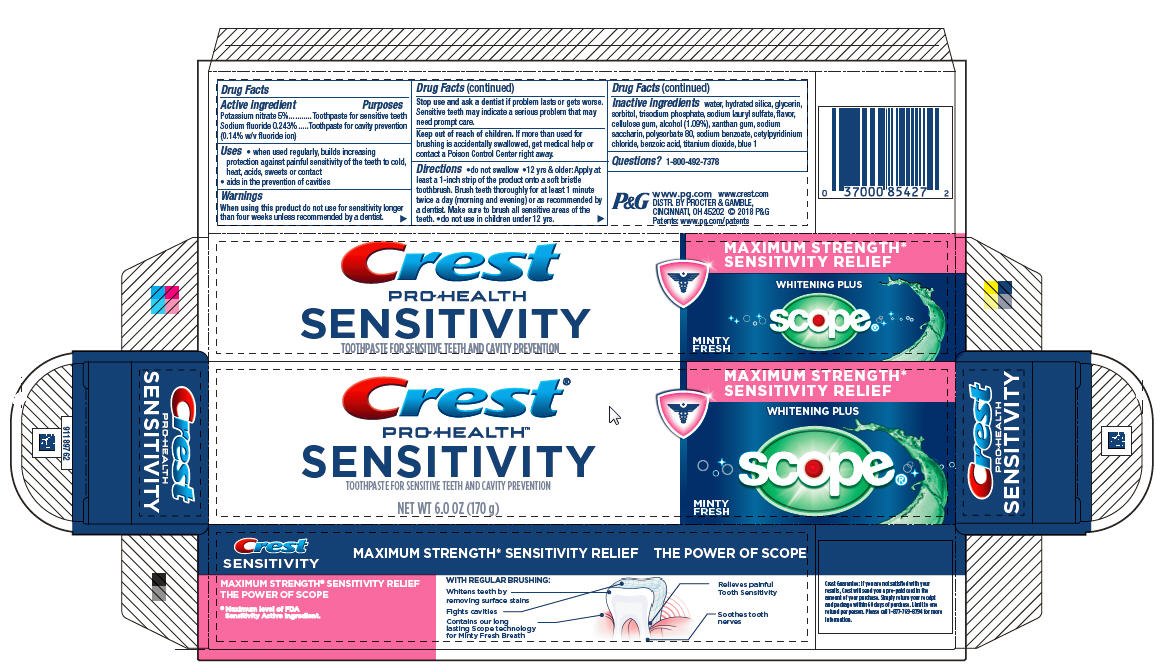
CREST SENSITIVITY WHITENING PLUS SCOPE- sodium fluoride and potassium nitrate paste, dentifrice
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Potassium nitrate 5% | Toothpaste for sensitive teeth |
| Sodium fluoride 0.243% (0.14% w/v fluoride ion) | Toothpaste for cavity prevention |
Uses
- when used regularly, builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- aids in the prevention of cavities
Warnings
When using this product do not use longer than four weeks unless recommended by a dentist.
Stop use and ask a dentist if problem lasts or gets worse. Sensitive teeth may indicate a serious problem that may need prompt care.
Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not swallow
- 12 yrs. & older: Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not use in children under 12 yrs.
Inactive ingredients
water, hydrated silica, glycerin, sorbitol, trisodium phosphate, sodium lauryl sulfate, flavor, cellulose gum, alcohol (1.09%), xanthan gum, sodium saccharin, polysorbate 80, sodium benzoate, cetylpyridinium chloride, benzoic acid, titanium dioxide, blue 1
Questions?
1-800-492-7378
Dist. by Procter & Gamble, Cincinnati, OH 45202
PRINCIPAL DISPLAY PANEL - 170 g Tube Carton
Crest®
PRO-HEALTH
SENSITIVITY
TOOTHPASTE FOR SENSITIVE TEETH AND CAVITY PREVENTION
NET WT 6.0 OZ (170 g)
MAXIMUM STRENGTH*
SENSITIVITY RELIEF
WHITENING PLUS
scope
®
MINTY FRESH