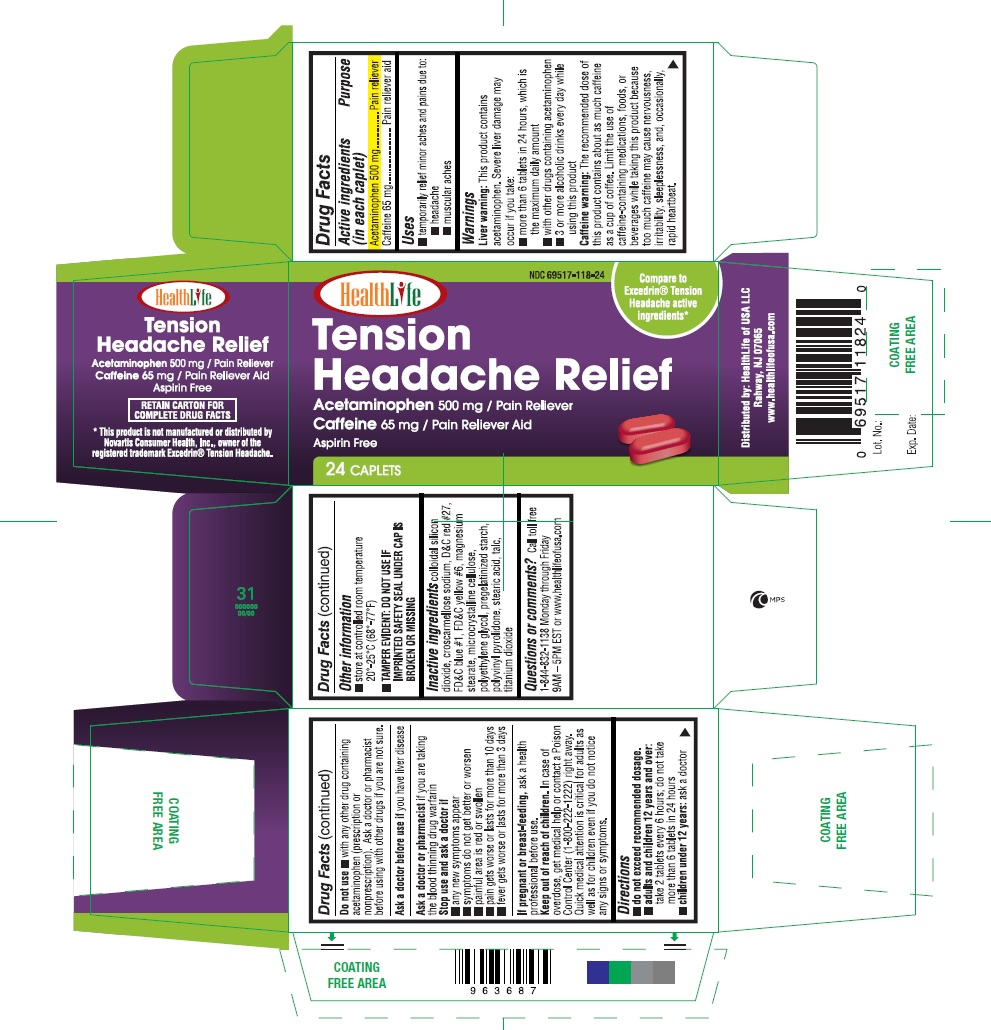
Warnings
Liver warning: This product contains acetaminophen. severe liver damage may occur if you take:
- more than 6 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally rapid heartbeat.
Do not Use
- with any other drug containing acetaminophen (prescription or nonprescription). Ask a doctor or pharmacist before using with other drugs if you are not sure.
Stop use and ask a doctor if
- Any new symptoms appear
- Symptoms do not get better or worsen
- Painful area is red or swollen
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
Keep out of reach of children.
In case of overdosage, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- Do not exceed recommended dosage.
- Adults and children 12 years and over: Take 2 tablets every 6 hours; do not take more than 6 tablets in 24 hours
- Children under 12 years: Ask a doctor
Other Information
- Store at controlled room temperature 20°-25°C (68°-77°F)
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP
Inactive Ingredients
- Colloidal silicon doixide
- Croscarmellose sodium
- D&C red # 27
- FD&C blue # 1
- FD&C yellow # 6
- Magnesium stearate
- Microcrystalline cellulose
- Polyethylene glycol
- Pregelatinized starch
- Polyvinyl pyrolidone
- Stearic acid
- Talc
- Titanium dioxide