Warnings
Do not use for children under 12 years of age unless directed by a doctor.
Do not take unless directed by a doctor if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Do not take if you are taking sedatives or tranquilizers, without first consulting your doctor.
Directions
- doage should be taken 1 hour before travel starts
| Adults and children 12 years and over |
take 1 or 2 tablets once daily or as directed by doctor |
Other information
- Tamper Evident: do not use if safety seal under cap is broken or missing
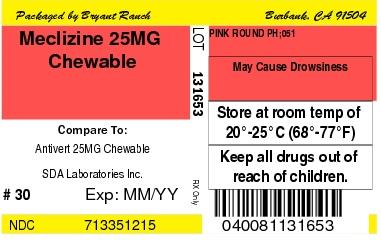
- store at room temperature 20°-25°C (68°-77°F)
Inactive ingredients
Croscarmellose sodium, dextrose, FD&C Red#40, flavor, magnesium stearate, microcrystalline cellulose, silicon dioxide, sodium saccharine, stearic acid
HOW SUPPLIED
Product: 71335-1215
NDC: 71335-1215-0 120 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-1 30 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-2 20 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-3 25 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-4 40 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-5 60 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-6 90 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-7 8 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-8 14 TABLET, CHEWABLE in a BOTTLE
NDC: 71335-1215-9 10 TABLET, CHEWABLE in a BOTTLE