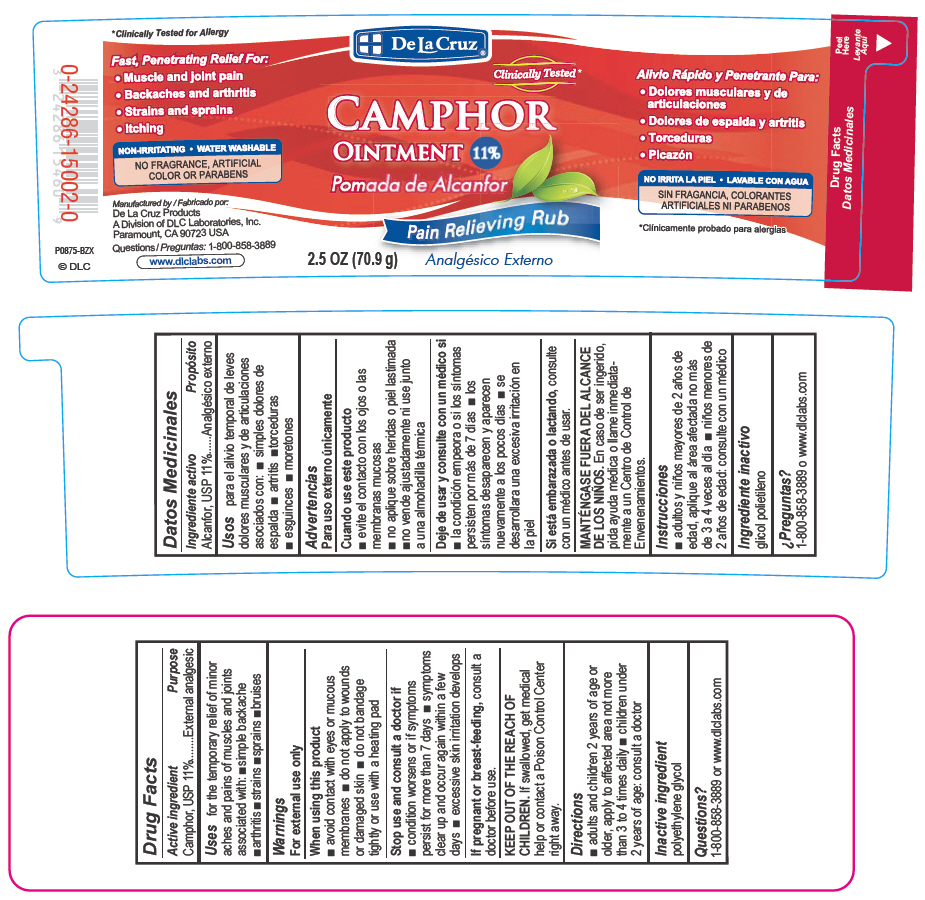
Uses
for the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains
When using this product
avoid contact with eyes or mucous membranes
do not apply to wounds or damaged skin
do not bandage tightly or use with a heating pad
Stop use and consult a doctor if
condition worsens or if symptoms persist for more than 7 days
symptoms clear up and occur again within a few days.
excessive skin irritation develops
KEEP OUT OF THE REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children 2 years of age or older, apply to affected area not more than 3 to 4 times daily
children under 2 years of age: consult a doctor
De La Cruz
CAMPHOR
Ointment 11%
Pain relieving rub
2.5 OZ (70.9g)
FAST, PENETRATING RELIEF FOR:
Muscle and joint pain
Backaches and arthritis
Strains and sprains
Itching
NON-IRRITATING
WATER WASHABLE
NO PARABENS OR ARTIFICIAL FRAGRANCES OR COLORS
Manufactured by:
De La Cruz Products
A Division of DLC Laboratories, Inc.
Paramount, CA 90723 USA
Questions: 1-800-858-3889
www.dlclabs.com (c) DLC