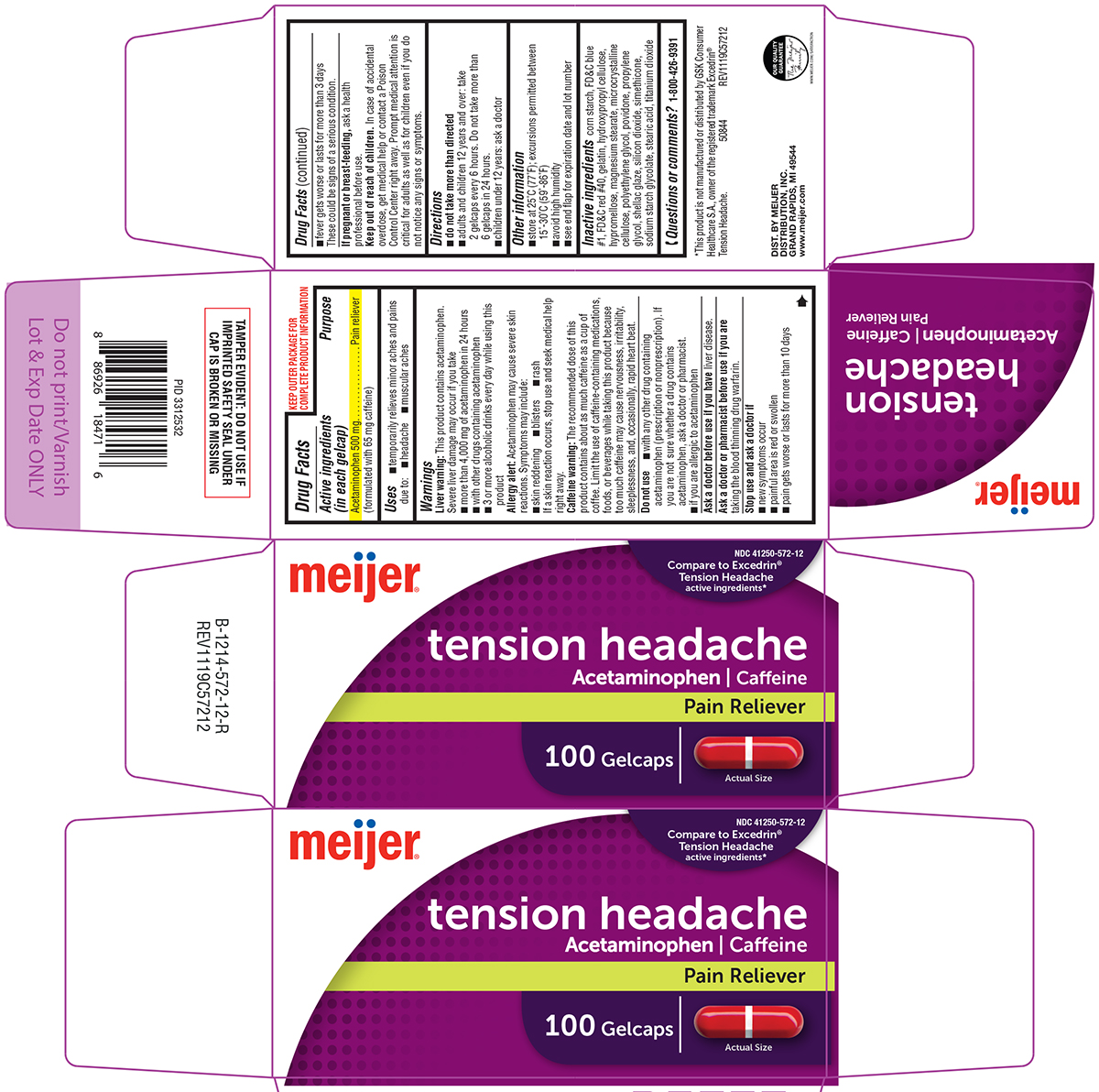
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen
Directions
- do not take more than directed
- adults and children 12 years and over: take 2 gelcaps every 6 hours. Do not take more than 6 gelcaps in 24 hours.
- children under 12 years: ask a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid high humidity
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, FD&C blue #1, FD&C red #40, gelatin, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, propylene glycol, shellac glaze, silicon dioxide, simethicone, sodium starch glycolate, stearic acid, titanium dioxide
Principal display panel
NDC 41250-572-12
Compare to Excedrin® Tension Headache active ingredients*
meijer®
tension headache
Acetaminophen ι Caffeine
Pain Reliever
100 Gelcaps
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by GSK Consumer Healthcare S.A., owner of the registered trademark Excedrin® Tension Headache.
50844 REV1119C57212
DIST. BY MEIJER
DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.com
Meijer 44-572