Active Ingredient
Benzocaine, 6% w/v
Purpose
Topical Analgesic
Active Ingredient
Isopropyl Alcohol, 60% w/v
Use(s)
For the temporary relief of pain and/or itching associated with minor burns, sunburn, minor cuts, scrapes, insect bites, or minor skin irritations.
Warnings
For External Use Only
• Flammable, keep away from fire or flame
• Avoid contact with eyes; if this occures, rinse thoroughly with water
• Do not use with electrocautery procedures
Stop use if
• Irritation and redness develop
• Condition worsens or symptoms persists for more than 7 days, or clear up and occur again within a few days, discontinue use and consult a doctor
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
-
For adults and children 2 years of age and older: Apply to affected area not more than 3-4 times daily.
-
Children under 2 years: Consult a physician before use
-
For other uses: Apply as needed
Other Information
• Store at room temperature between 15º-30ºC (59º-86ºF)
• Avoid excessive heat
Inactive Ingredients
Water
Questions?
1-888-396-2739 Monday - Friday 9AM-5PM EST.
Label
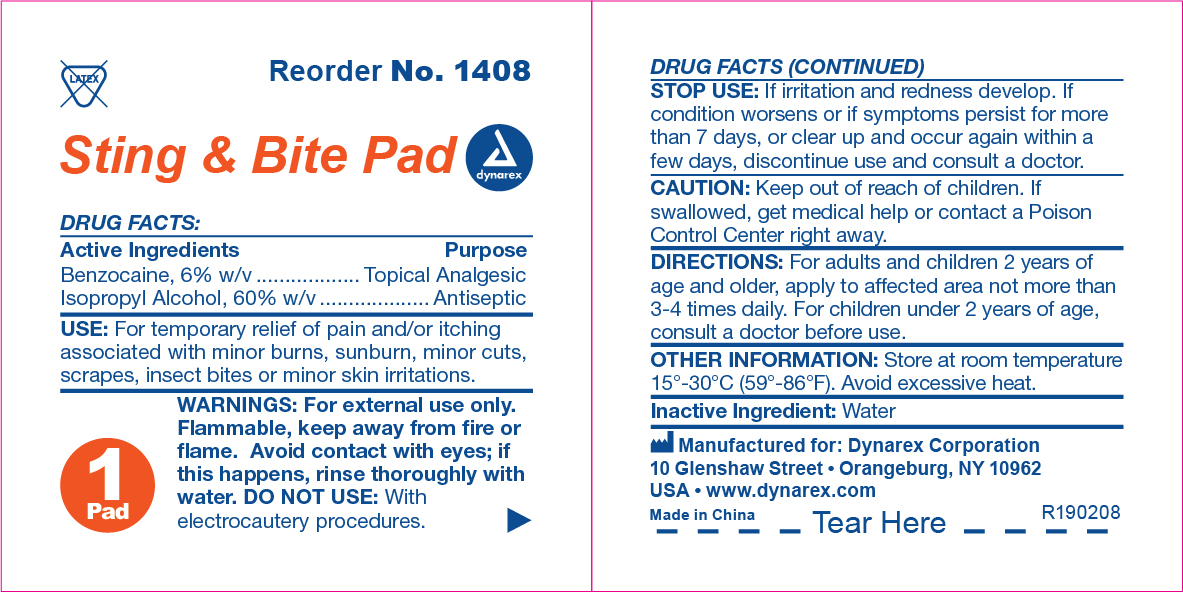 Sting & Bite Pad Label
Sting & Bite Pad Label
Label 1408UB-10
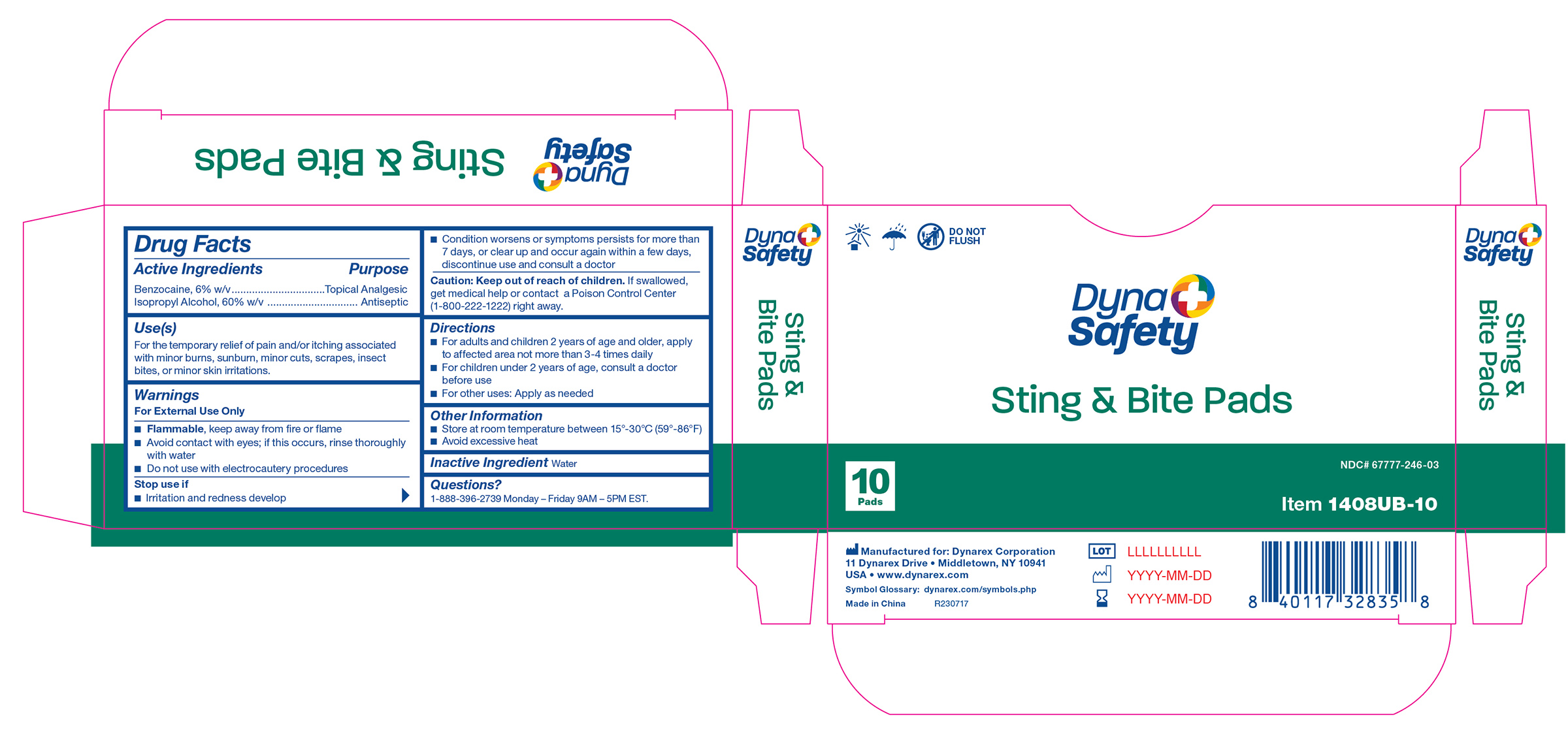 1408UB-10
1408UB-10
 Sting & Bite Pad Label
Sting & Bite Pad Label
 1408UB-10
1408UB-10