COMPONENT E-S WITH TYLAN- progesterone, estradiol benzoate, tylosin tartrate implant
Elanco US Inc.
----------
Elanco™
Component™
E-S with Tylan
(progesterone and estradiol and tylosin implant)
STEERS
Each implant consists of 200 mg progesterone USP
and 20 mg estradiol benzoate and 29 mg tylosin tartrate
STEER IMPLANTS
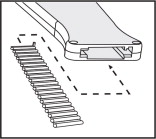
20 dose Cartridge Belt
Use with a Component Implanter
For Use In Animals Only
DESCRIPTION:
Each cartridge belt holds 20 doses of Component E-S with Tylan (progesterone and estradiol and tylosin implant) Implants. Each dose of 9 pellets consists of 8 pellets containing a total of 200 mg progesterone USP and 20 mg estradiol benzoate plus 1 pellet containing 29 mg tylosin tartrate as a local antibacterial. Component E-S (progesterone and estradiol implant) Implants are recommended for use in steers weighing 400 lbs or more for INCREASED RATE OF WEIGHT GAIN AND IMPROVED FEED EFFICIENCY.
Do not use in veal calves. Effectiveness and animal safety in veal calves have not been established.
GENERAL INSTRUCTIONS:
Study the instructions which should be followed carefully at all times, avoiding short cuts. Skin infection can be avoided by properly preparing implant site and implanter. During fly season use fly repellent on implant site. One designated team member should always do the implanting. Cleanliness of hands and instruments is important at all times.
WARNING:
Not to be used in animals intended for subsequent breeding, or in dairy animals. Implant one dose — 9 pellets (entire contents of one cartridge cell) — in the ear subcutaneously. Any other site of implantation is in violation of Federal Law. DO NOT attempt to salvage implantation site for animal feed or human food.
A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
RESTRICTED DRUG (CALIFORNIA) — USE ONLY AS DIRECTED — FOR USE IN ANIMALS ONLY — NOT FOR HUMAN USE — KEEP OUT OF REACH OF CHILDREN
CAUTION:
Bulling, rectal prolapse, ventral edema and elevated tail-heads have occasionally been reported in animals implanted with progesterone and estradiol benzoate.
IMPLANTING INSTRUCTIONS:
Restrain the Animal
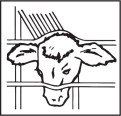
Speed of implantation as well as safety of handlers is best achieved by restraining animal in a squeeze chute using head restraint. When implanting horned cattle, better control is obtained with additional use of nose tongs.
Prepare the Implant Site
Scrub the back side of the ear (implant site) with a piece of clean absorbent cotton which has been soaked with topical germicidal solution. Follow manufacturer's directions on germicide for correct strength and preparation of solution. Avoid getting disinfectant in animal's eyes.
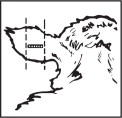
Where to Implant
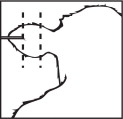
The full contents of one cartridge cell should be implanted beneath the skin on the back side of the middle one-third of the ear as illustrated in the drawing. The implant must not be closer to the head than the edge of the auricular cartilage ring farthest from the head. The location for insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site. Avoid injuring the large arteries, veins and cartilage of the ear.
Insert the Needle
With one hand firmly grasp the ear. With other hand insert needle point through the skin and ease forward on a lateral plane until the entire length of the needle is under the skin.
Implant the Pellets
After inserting the needle fully in the correct position, squeeze the trigger fully as the needle is withdrawn from the ear. This properly deposits the implant in the needle track. This procedure should prevent breakage or crushing of pellets if otherwise forced into contact with tough fibrous-tissue underlying the skin. The length and total contact area of a single dose are designed to permit absorption of the hormones after implantation to stimulate good weight gain. Broken or crushed pellets may interfere with rates of gain and may lead to undesirable side effects such as noted in the CAUTION.
Storage Conditions
Store at controlled room temperature 15° to 30° C (59° to 86° F)
DO NOT refrigerate – avoid excessive heat and humidity. Discard open foil pouches.
Approved by FDA under NADA #110-315
Manufactured by a non-sterilizing process.
Component E-S with Tylan (progesterone and estradiol and tylosin implant) is covered by U.S. Patent No. 5,874,098
Component, Tylan, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
Distributed by Elanco US, Inc., Greenfield, IN 46140, USA
Tylosin: Product of the United Kingdom
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US, Inc. at 1-888-545-5973. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
Revised: April 2020
PA101948X AH0328
Principal Display Panel - Component E-S With Tylan Cartridge Label
Elanco™
Component™
™
E-S with Tylan™
(progesterone and estradiol and tylosin implant)
STEERS
| COMPONENT E-S WITH TYLAN
progesterone, estradiol benzoate, tylosin tartrate implant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Elanco US Inc. (966985624) |