PED X CALLUS REMOVERS, EXTRA THICK MEDICATED- salicylic acid patch
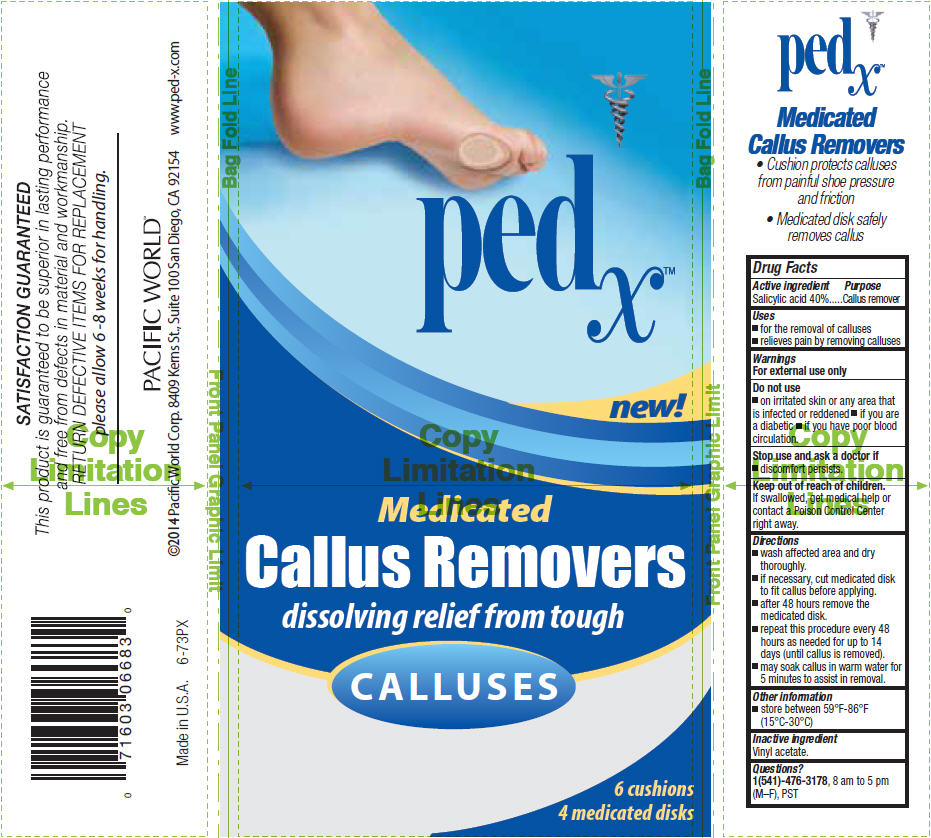
PED X CALLUS REMOVERS, MEDICATED- salicylic acid patch
Pacific World Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic acid 40%
Uses
- for the removal of calluses
- relieves pain by removing calluses
Warnings
For external use only
Do not use
- on irritated skin or any area that is infected or reddened
- if you are a diabetic
- if you have poor blood circulation.
Stop use and ask a doctor if
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly.
- if necessary, cut medicated disk to fit callus before applying.
- after 48 hours remove the medicated disk.
- repeat this procedure every 48 hours as needed for up to 14 days (until callus is removed).
- may soak callus in warm water for 5 minutes to assist in removal.
Other information
- store between 59°F-86°F (15°C-30°C)
Inactive ingredient
Vinyl acetate.
Questions?
1(541)-476-3178, 8 am to 5 pm (M–F), PST
PRINCIPAL DISPLAY PANEL - 4 mg Patch Cello Pack - Extra Thick
pedx™
new!
Extra Thick Medicated
Callus Removers
dissolving relief from tough
CALLUSES
6 cushions
4 medicated disks
PRINCIPAL DISPLAY PANEL - 4 mg Patch Cello Pack - Medicated
pedx™
new!
Medicated
Callus Removers
dissolving relief from tough
CALLUSES
6 cushions
4 medicated disks