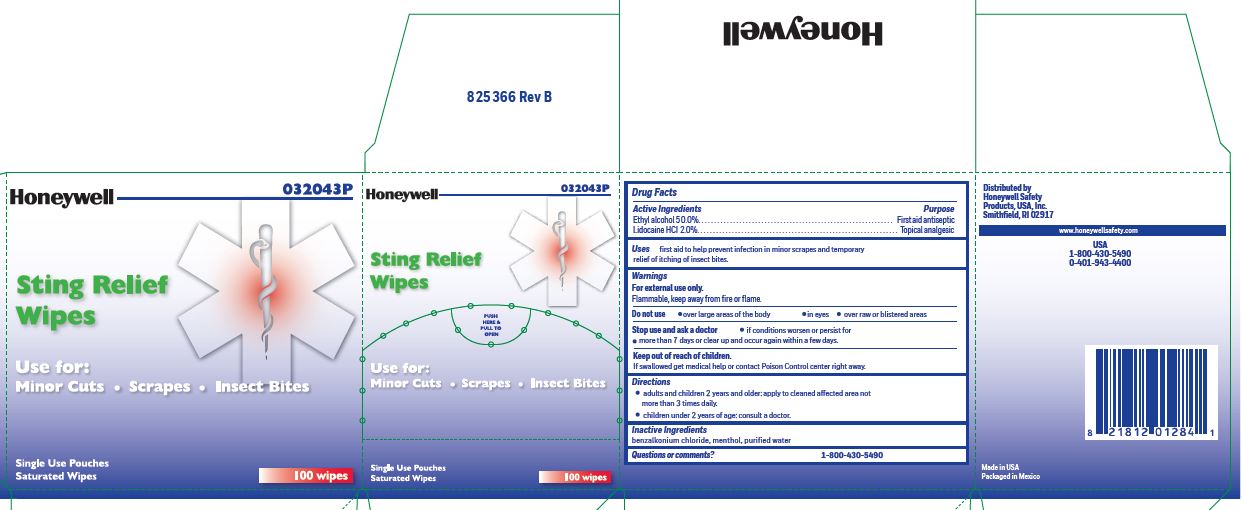
STING RELIEF PAD- ethyl alcohol, lidocaine swab
NOX-A-STING- ethyl alcohol, lidocaine swab
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active Ingredients
in each wipe:
Ethyl alcohol 50.0%
Lidocaine HCl 2.0%
Purpose
Antiseptic
Topical pain relief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Warnings
- For external use only
- Flammable, keep away from open fire or flame
Do not use
- over large areas of the body
- in eyes
- over raw or blistered areas
Stop use and ask a doctor
- if conditions worsen or persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
Inactive ingredients
benzalkonium chloride, menthol, and purified water
Questions or comments?
1-800-430-5490
Sting Relief label
Nox-A-Sting label
