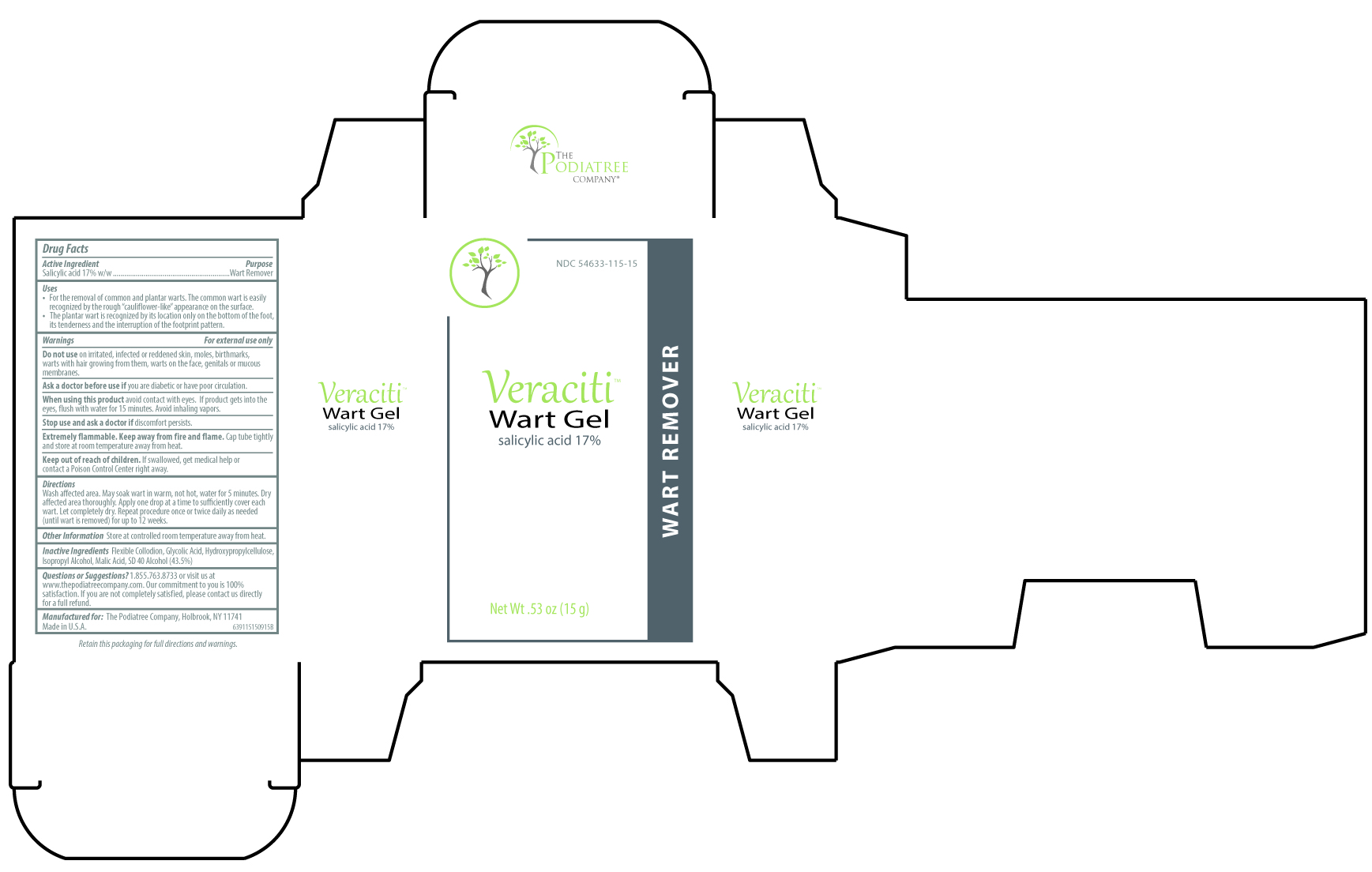
Uses
- For the removal of common and plantar warts. The common wart is easily recognized by the rough “cauliflower-like” appearance on the surface.
- The plantar wart is recognized by its location only on the bottom of the foot, its tenderness and the interruption of the footprint pattern.
Warnings
For external use only.
Do not use
on irritated, infected or reddened skin, moles, birthmarks, warts with hair growing from them, warts on the face, genitals or mucous membranes.
When using this product
avoid contact with eyes. If product gets into the eye, flush with water for 15 minutes. Avoid inhaling vapors.
Directions
Wash affected area. May soak wart in warm, not hot, water for 5 minutes. Dry affected area thoroughly. Apply one drop at a time to suffciently cover each wart. Let completely dry. Repeat procedure once or twice daily as needed (until wart is removed) for up to 12 weeks.
Inactive ingredients
Flexible Collodion, Glycolic Acid, Hydroxypropylcellulose, Isopropyl Alcohol, Malic Acid, SD 40 Alcohol (43.5%)