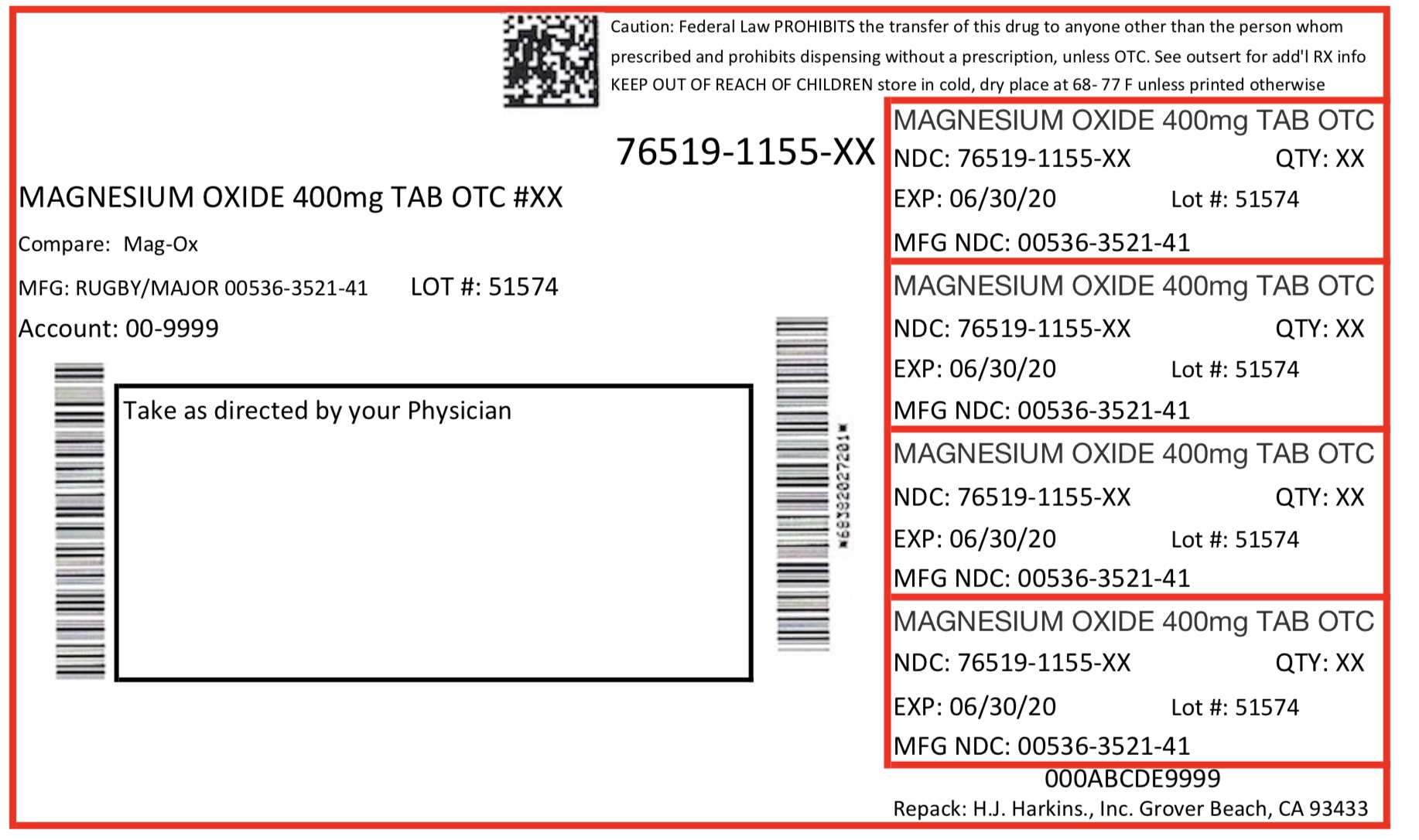
KEEP OUT OF REACH OF CHILDREN
Ask a doctor before use if
you have kidney disease
you are taking a prescription drug (antacids may interact with certain prescription drugs)
you are pregnant or breast feeding.
Do not take more than 2 tablets in a 24 hour period.
May have a laxative effect.
Keep out of reach of children.
Dosage and Administration
take one or two antacid tablets daily. Do not exceed two tablets unless directed by a physician.
Storage & Handling
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Tamper evident, do not use if imprinted safety seal under cap is broken or missing.
Inactive Ingredients
Croscarmellose Sodium, Microcrystalline Cellulose, Silicon Dioxide, and Stearic Acid.
Warnings
Do not take this product if you are presently taking a prescription drug without consulting your physician or other health care professional. If you have kidney disease, take only under the supervision of a physician. May have a laxative effect. If pregnant or breast-feeding, ask a health professional before use.
Pregnancy or Breast Feeding
If pregnant or breast-feeding, ask a health professional before use.
Keep Out of Reach of Children