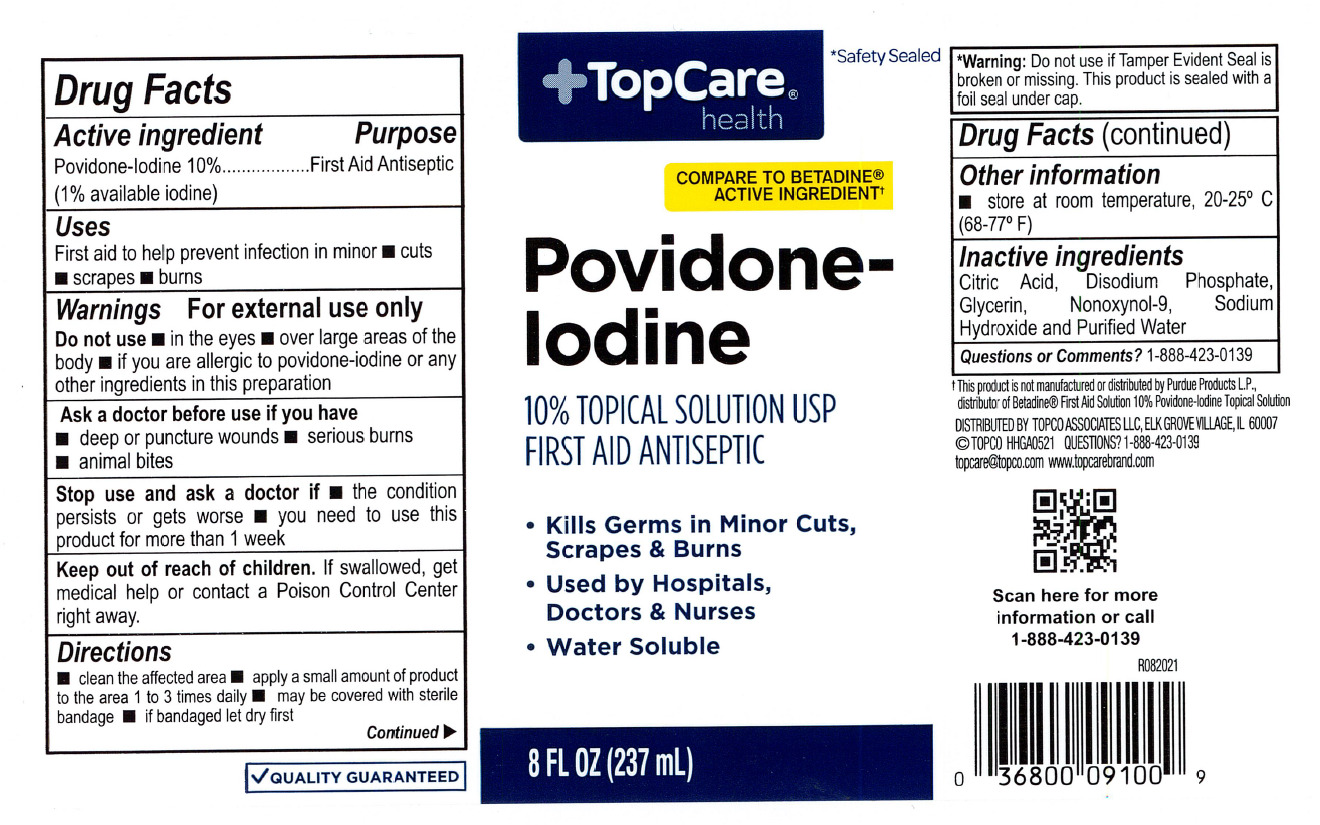
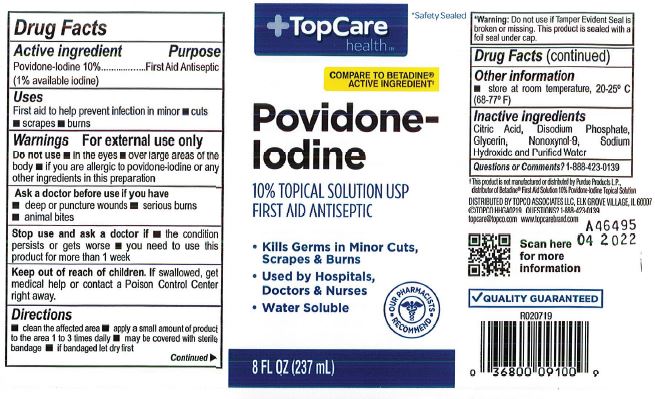
Active Ingredient
Povidone-Iodine 10%
(1% available iodine)
Purpose
First Aid Antiseptic
Use
First aid antiseptic to prevent infection in minor cuts and burns.
Warnings
For External Use Only
Ask a doctor before use if you have
- deep punture wounds
- animal bites
- serious burns
Stop use and consult a doctor if
- the condition persists or gets worse
- irritation and redness develop and persits for more than 72 hours
When using this product do not
- use in eyes
- use on individuals who are allergic or sensitive to iodine or use for longer thn 1 week unless directed by a doctor
- apply over large areas of the body
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
Clean the affected area. Apply a small amount on the area 1 to 3 times daily. May be covered with sterile bandage. If badaged, let it dry first.
Other information
store at room temperature, 20-25C (68-77F)
Inactive Ingredients
Citric Acid, Disodium Phosphate, Glycerin, nonoxynol-9. Sodium Hydroxide and Purified Water.
Sunmark Label
8 fl oz

16 fl oz

Good Neighbor Label


Leader Label


Quality Choice Label


Select Brand Label

Publix Label

Premier Value Label

Fred's Label

DDM Label

Rite Aid Label


CVS Label

Vida MIaa Label

HEB Label

Health Mart Label


Purdue Betadine Label

Top Care Label
Humco Holding Group, Inc.






















