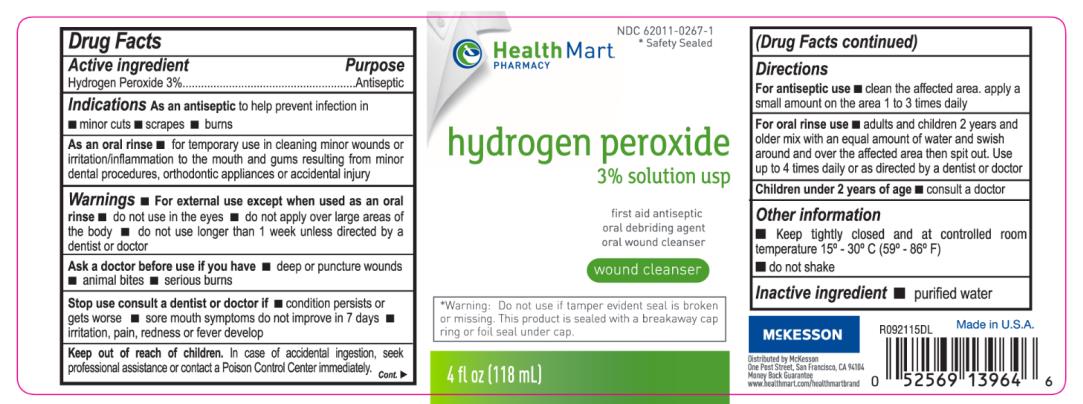
As an oral rinse for temporary use in cleaning of minor wounds or irritation/inflammation to the mouth and gums resulting from minor dental procedures, orthodontic appliances or accidental injury.
Warnings
For external use except when used as an oral rinse.
Do not use in the eyes.
Do not apply over large areas of the body.
Do not use longer than 1 week unless directed by a dentist or doctor.
Stop use consult a dentist or doctor if:
condition persists or gets worse, sore mouth symptoms do not improve in 7 days, irritation, pain, redness or fever develop.
Keep out of reach of children.
In case of acciedntal ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
For antiseptic use: Clean the affected area, apply a small amount on the area 1 to 3 times daily.
For oral rinse use: Adults and children 2 years and older mix with an equal amount of water and swish around and over the affected area then spit out. Use up to 4 times daily or as directed by a dentist or a doctor.
Children under 2 years of age: consult a doctor.