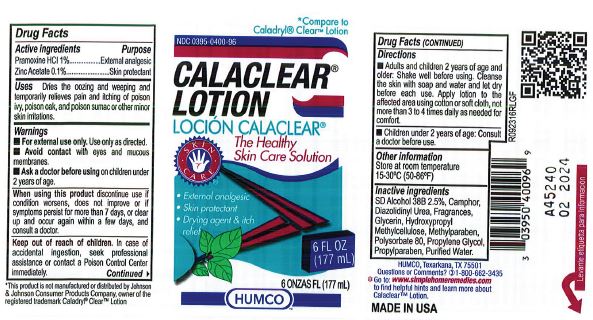
CALACLEAR- pramoxine hydrochloride and zinc acetate lotion
Humco Holding Group, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Humco Calamine Lotion
Drug Facts
Active Ingredient
Pramoxine HCl 1%
Purpose
External Analgesic
Active Ingredient
Zinc Acetate 0.1%
Use
Dries the oozing and weeping and temporarily relieves pain and itching of poison ivy, poison oak, and poison sumac or other minor skin irritations.
Warnings
For external use only. Use only as directed.
Avoid contact with eyes and mucous membranes.
Ask a doctor before using
on children under 2 years of age.
When using this product. Discontinue use if
condition worsens, does not improve or if symptoms persist for more than 7 days, or clear up and occur again within a few days. and consult a doctor.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- Adults and children 2 yrs. of age and older. Shake well before using. Cleanse the skin with soap and water and let dry before each use. Apply lotion to the affected area using cotton or soft cloth, not more than 3 to 4 times daily as needed for comfort.
- Children under 2 years of age: Consult a doctor before use.
Other Information
- store at room temperature 15
o – 30
oC (50
o – 85
oF)
Inactive Ingredient
SD Alcohol 38B 2.5%, Camphor, Diazolidinyl Urea, Fragrances, Glycerin, Hydroxypropyl Methylcellulose, Methylparaben, Polysorbate 80, Propylene Glycol, Propylparaben, Purified Water.
GNP Label

Leader Label

Quality Choice Label

Sunmark Label

Publix Label

Premiere Value Label

DDM Label

CVS Label


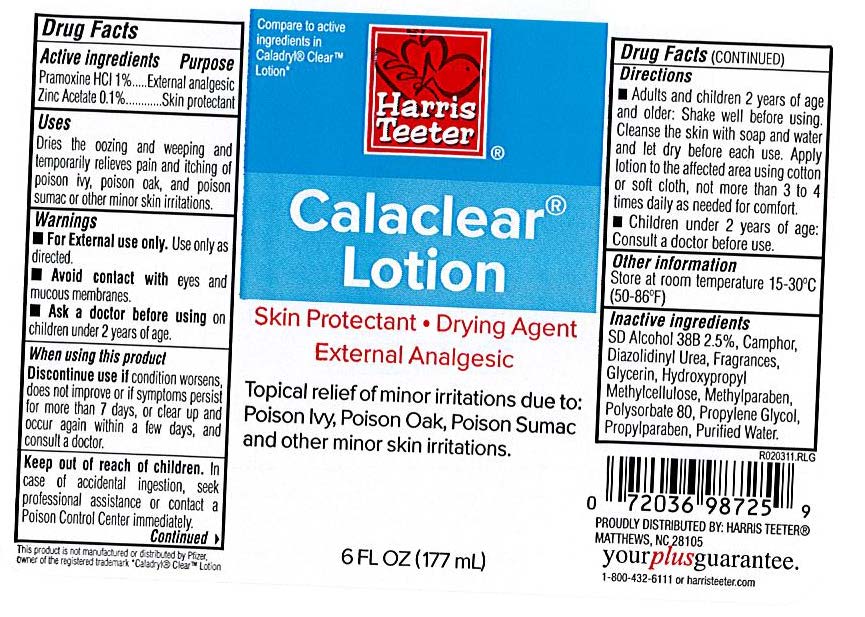
Harris Teeter Label
Best Choice Label

HEB Label

Equate Label


WM Label
RA Label

Top Care Label

Humco Holding Group, Inc.
















