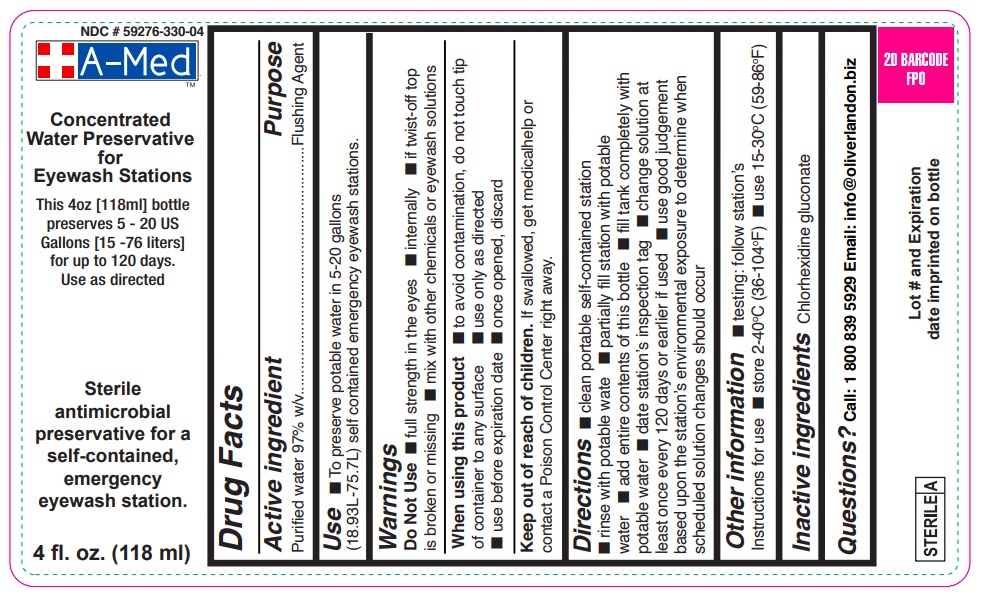
A-MED- eyewash additive solution, concentrate
Oliver Landon Intl Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Purified water USP 97% W/V
Use for flushing the eye to remove loose foreign material.
Warnings
For external use only
Do not use
- •
- if solution changes color or becomes cloudy
- •
- if you have open wounds in or near the eyes. Get medical help right away.
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- do not reuse; once opened, discard
Stop use and ask a doctor if
- •
- continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Inactive ingredients Chlorhexidine gluconate
Distributed by: Oliver Landon Intl Inc.
21 Pine Road, Belleville, St. Michael
BB 11113 Barbados
Questions or concerns:
email - info@oliverlandon.biz or call (800) 839-5929
Made in U.S.A.
Store out of direct sunlight.
A-Med Wash
NDC 59276-330-04
Eyewash Additive Concentrate
Antimicrobial preservative for a self-contained, emergency eyewash station.
Preserves from 5 – 20 US Gallons (18.9 – 75.7 Liters)
4 fl. oz. (118 ml)
Oliver Landon Intl Inc.