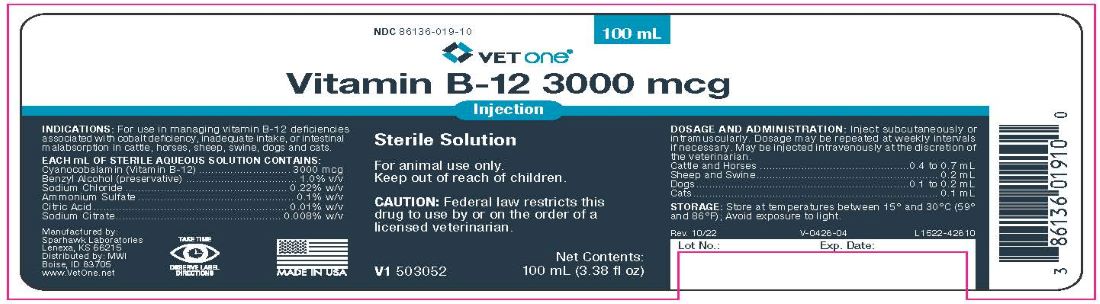
3000 mcg/mL
Sterile Solution
For animal use only.
Keep out of reach of children.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
INDICATIONS
For use as a supplemental nutritive source of vitamin B12 in cattle, horses, swine, sheep, dogs and cats.
CONTAINS
Each mL of sterile aqueous solution contains:
Cyanocobalamin (B12)..........3000 mcg
With sodium chloride 0.22% w/v, ammonium sulfate 0.1% w/v, citric acid 0.01% w/v, sodium citrate 0.008% w/v, and benzyl alcohol 1.0% v/v (preservative).
STORAGE
Store at controlled room temperature between 15°C and 30°C (59°F - 86°F).
Avoid exposure to light.
AVOID EXPOSURE TO LIGHT
DOSAGE AND ADMINISTRATION
Inject subcutaneously or intramuscularly. Dosage may be repeated at weekly intervals if necessary. May be injected intravenously at the discretion of the veterinarian.
Cattle and Horses ...............................................0.4 to 0.7 mL
Sheep and Swine......................................................... 0.2 mL
Dogs...................................................................0.1 to 0.2 mL
Cats............................................................................. 0.1 mL
TAKE TIME OBSERVE LABEL DIRECTIONS