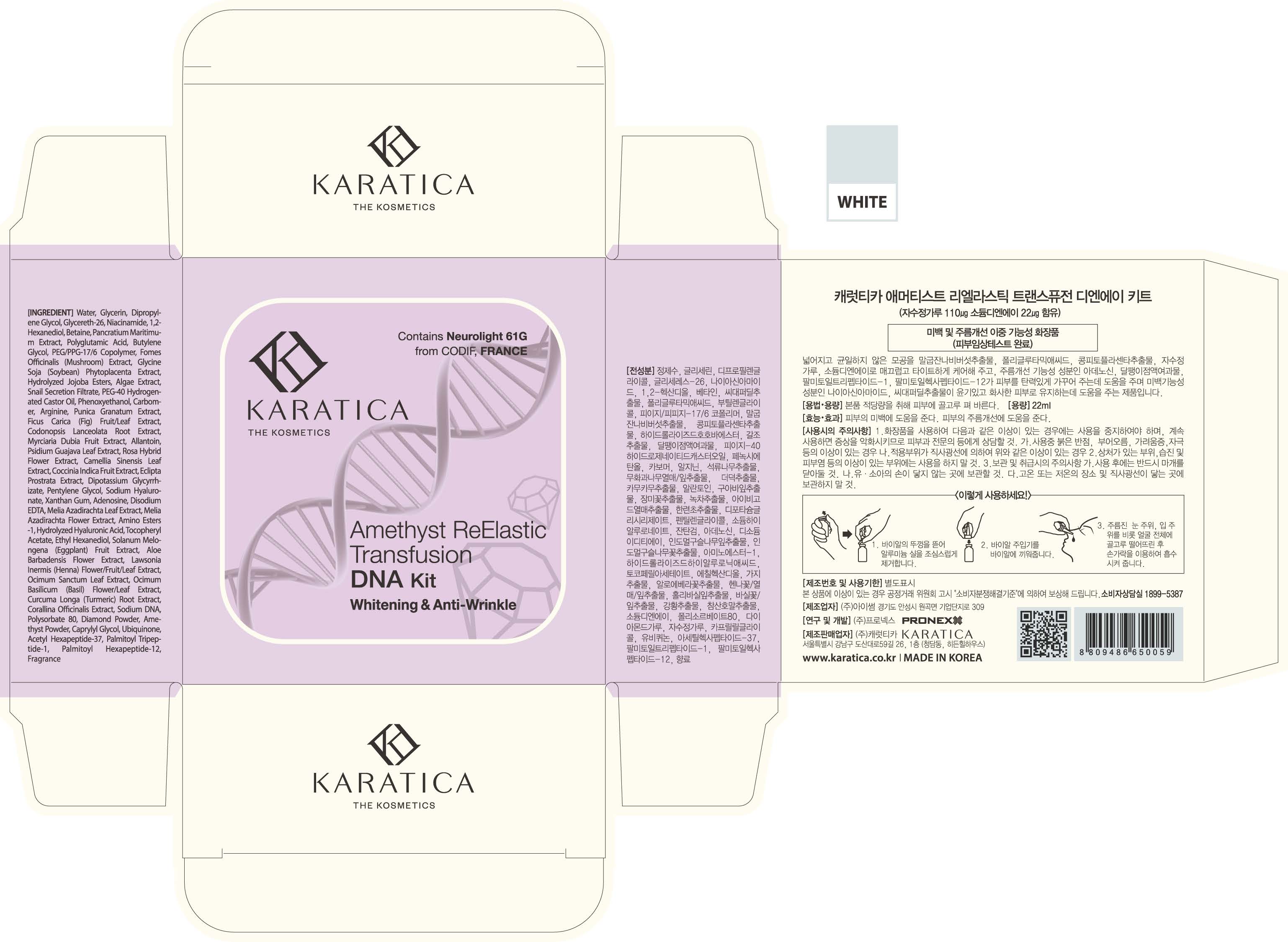
Take appropriate amount and evenly apply it to the skin
- Open the lid of the vial and remove aluminum thread carefully.
- Insert vial inserter in the vial.
- Drop the formula on wrinkles around eyes and mouth and the entire face. Then use your fingertips to help formula to be absorbed into the skin.
If you experience following symptoms after using the cosmetics, you should immediately stop using the cosmetics. If you continue to use them, the symptoms may worsen. Consult with your dermatologist.
A) When you experience red spots, swelling, itchiness and irritation while applying the product.
B) When the applied area experience symptoms while it is exposed to direct sunlight
2. Do not use the cosmetics on the areas where you have wounds, eczema or dermatitis
3. Precautions when storing and using the product
A) Keep the lid closed after using the product
B) Store it out of reach of children
C) Do not store it in places with high or low temperature or where it is exposed to the sunlight directly.