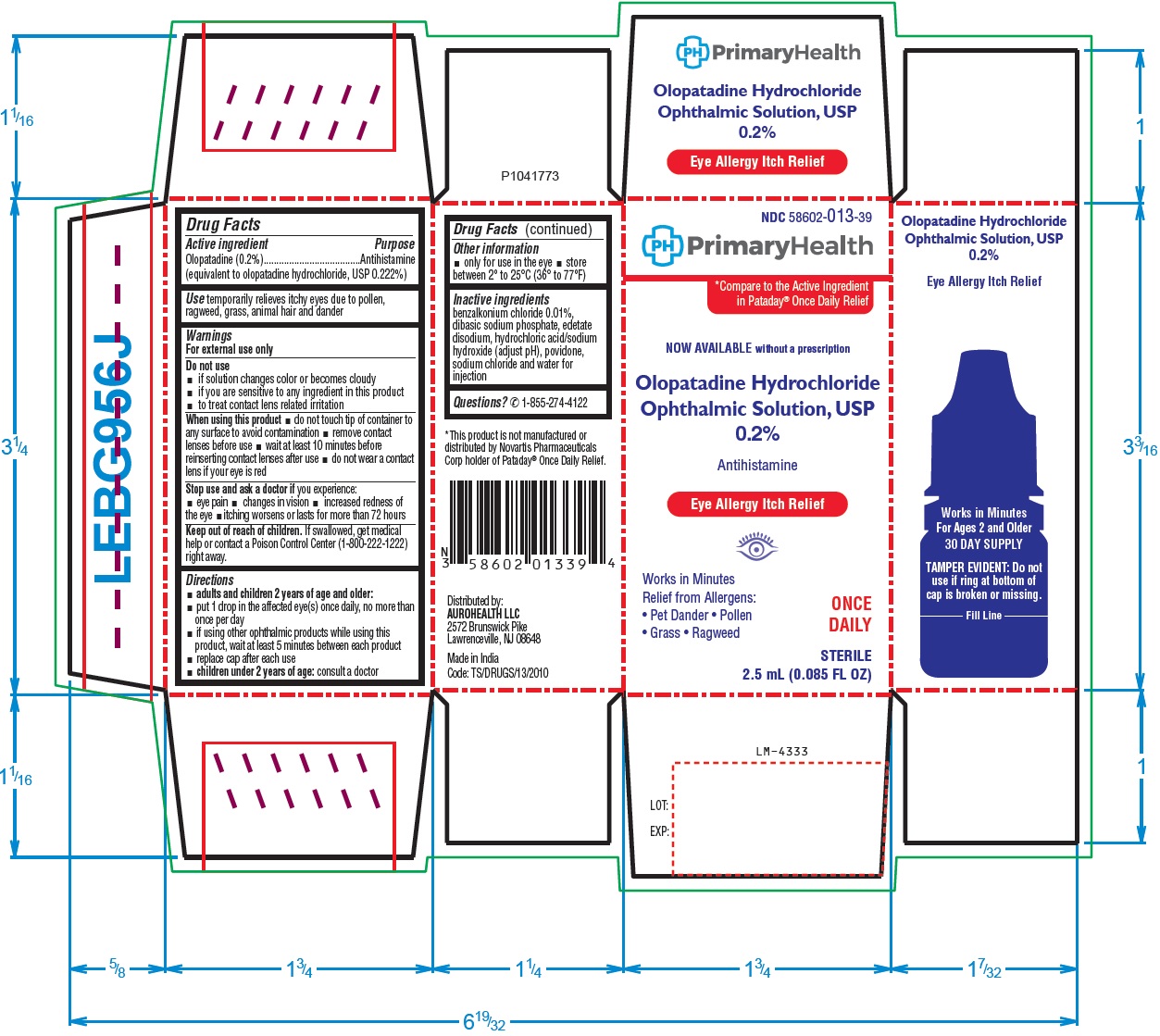
Drug Facts
Active ingredient
Olopatadine (0.2%)
(equivalent to olopatadine hydrochloride, USP 0.222%)
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) once daily, no more than once per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age:
consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate, edetate disodium, hydrochloric acid/sodium hydroxide (adjust pH), povidone, sodium chloride and water for injection
Questions?
✆1-855-274-4122
Distributed by:
AUROHEALTH LLC
2572 Brunswick Pike
Lawrenceville, NJ 08648
Made in India
Code: TS/DRUGS/13/2010
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.2% (2.5 mL Container)
PrimaryHealth NDC 58602-013-39
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.2%
Antihistamine
Eye Allergy Itch Relief
STERILE 2.5 mL (0.085 FL OZ)

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.2% (2.5 mL Container Carton)
NDC 58602-013-39
PrimaryHealth
*Compare to the Active Ingredient
in Pataday® Once Daily Relief
NOW AVAILABLE without a prescription
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.2%
Antihistamine
Eye Allergy Itch Relief
Works in Minutes
Relief from Allergens:
• Pet Dander • Pollen ONCE
• Grass • Ragweed DAILY
STERILE
2.5 mL (0.085 FL OZ)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.2% (2.5 mL Container Carton) Twin Pack
Twin Pack
NDC 58602-013-41
PrimaryHealth
*Compare to the Active Ingredient
in Pataday® Once Daily Relief
NOW AVAILABLE without a prescription
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.2%
Antihistamine
Eye Allergy Itch Relief
Works in Minutes
Relief from Allergens:
• Pet Dander • Pollen ONCE
• Grass • Ragweed DAILY
STERILE
Two 2.5 mL Bottles
(0.085 FL OZ EACH)
