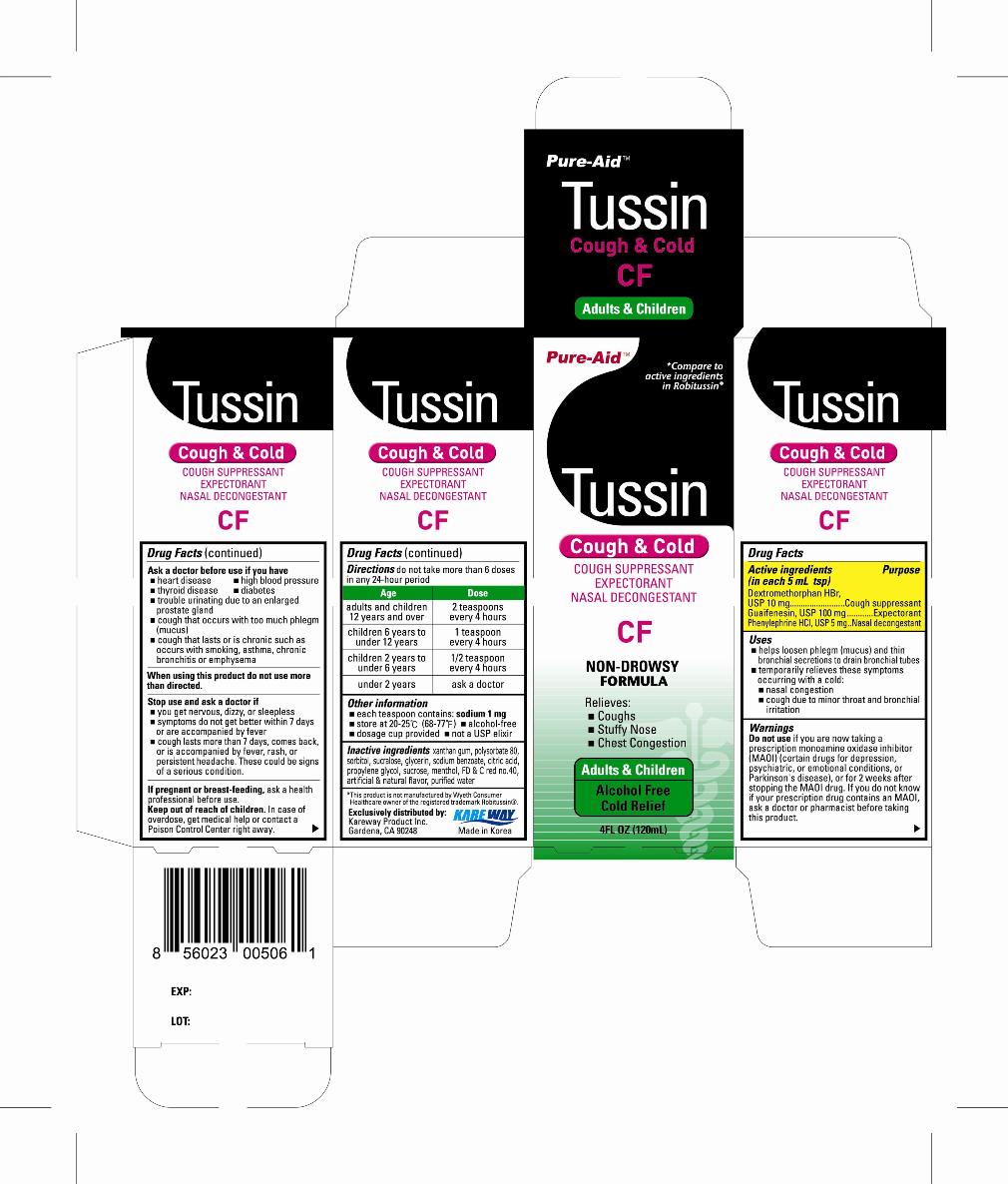
Active ingredients
(in each 5mL tsp)
Dextromethorphan HBr, USP 10mg
Guaifenesin, USP 100mg
Phenylephrine HCl, USP 5mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
- temporarily relieves these symptoms occurring with a cold:
-
- nasal congestion
- cough due to minor throat and bronchial irritation
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
| Age | Dose |
| adults and children 12 years and over | 2 tsp every 4 hours |
| children 6 years to under 12 years | 1 tsp every 4 hours |
| children 2 years to under 6 years | 1/2 tsp every 4 hours |
| children under 2 years | ask a doctor |
Other information
- each teaspoon contains:sodium 1mg
- store at 20-25°C (68-77°F)
- alcohol-free
- dosage cup provided
- not a USP elixir