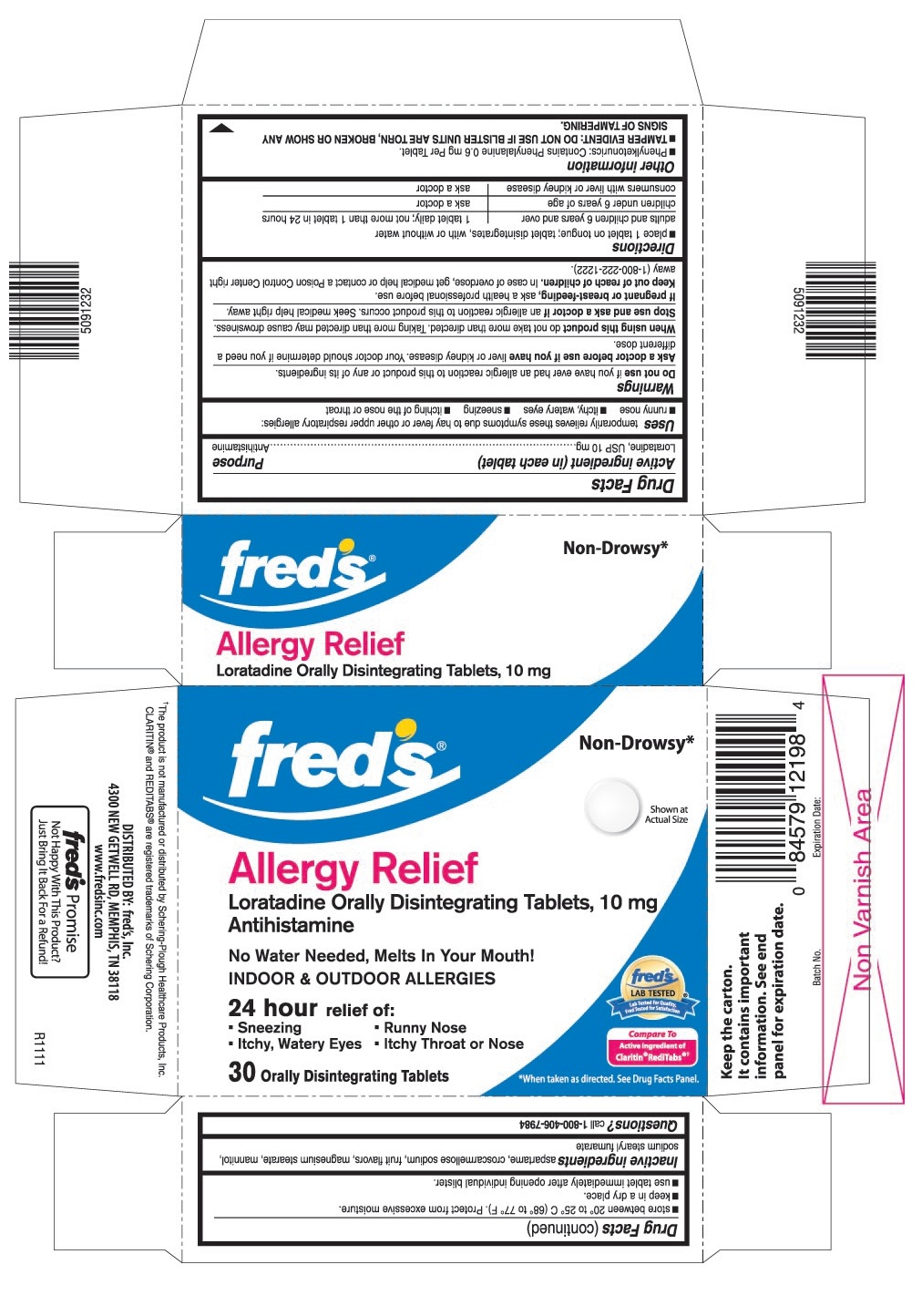
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
OTHER INFORMATION
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- keep in a dry place.
- use tablet immediately after opening individual blister.
INACTIVE INGREDIENTS
Aspartame, croscarmellose sodium, fruit flavors, magnesium stearate, mannitol, sodium stearyl fumarate
PRINCIPAL DISPLAY PANEL
Loratadine Orally Disintegrating Tablets, 10 mg
No Water Needed, Melts In Your Mouth!
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Compare To Active Ingredient of Claritin® RediTabs®†
30 Orally Disintegrating Tablets