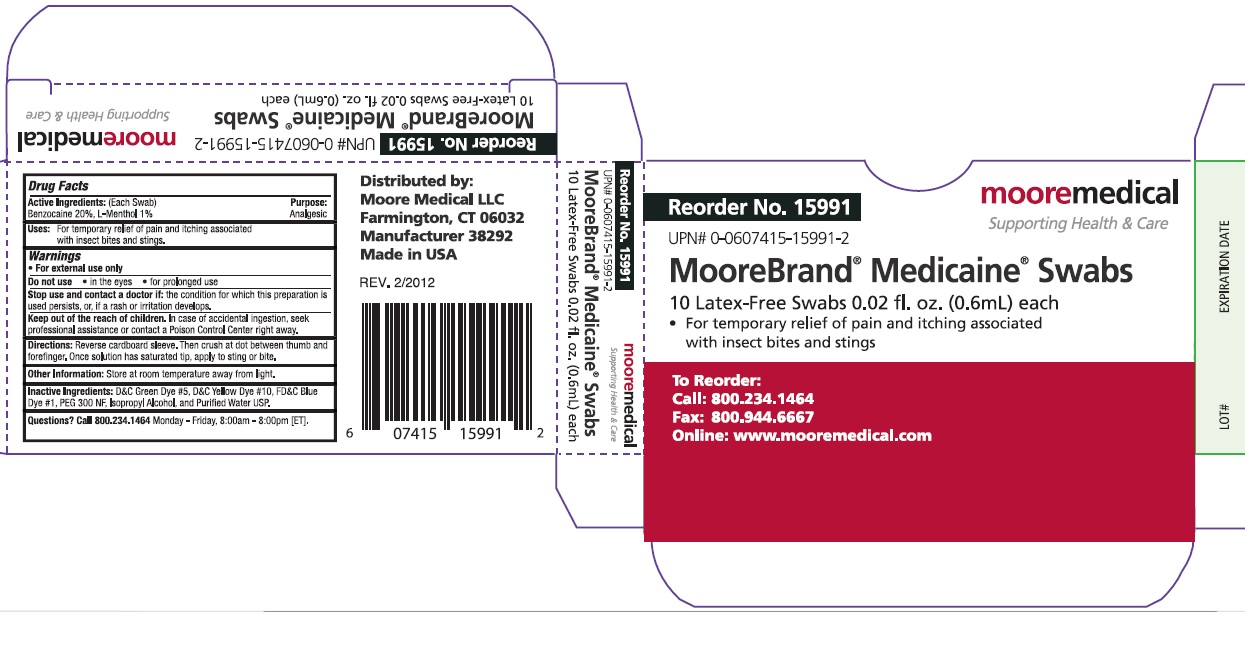
Active Ingredients: (Each Swab)
Benzocaine 20%, Menthol 1%
Uses:
For temporary relief of pain and itching associated with insect bites and stings.
Warnings
For external use only
Do not use
- in eyes
- for prolonged use
Stop use and contact a doctor if: the condition for which this preparation is used persists, or, if a rash or irritation develops.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away.
Directions: Reverse cardboard sleeve. Then crush at dot between thumb and forefinger. Once solution has saturated tip, apply to sting or bite.
Other Information: Store at room temperature away from light.
Inactive ingredients: D&C Green Dye #5, D&C Yellow dye #10, FD&C Blue Dye #1, Peg 300 NF, Isopropyl Alcohol and Purified Water USP.
Questions? Call 800-234-1464 Monday – Friday, 8:00am – 8:00pm [ET].
Reorder No. 15991
Moore Medical
Supporting Health & Care
UPN# 0-0607415-15991-2
Moore Brand Medicaine Swabs
10 Latex-Free Swabs 0.02 fl. Oz. (0.6mL) each
For temporary relief of pain and itching associated with insect bites and stings