FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment of EGFR Mutation-Positive Non-Small Cell Lung Cancer (NSCLC)
TAGRISSO is indicated as adjuvant therapy after tumor resection in adult patients with non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)].
1.2 First-line Treatment of EGFR Mutation-Positive Metastatic NSCLC
TAGRISSO is indicated for the first-line treatment of adult patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)].
1.3 First-line Treatment of EGFR Mutation-Positive Locally Advanced or Metastatic NSCLC
TAGRISSO in combination with pemetrexed and platinum-based chemotherapy is indicated for the first-line treatment of adult patients with locally advanced or metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)].
1.4 Previously Treated EGFR T790M Mutation-Positive Metastatic NSCLC
TAGRISSO is indicated for the treatment of adult patients with metastatic EGFR T790M mutation-positive NSCLC, as detected by an FDA-approved test, whose disease has progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy [see Dosage and Administration (2.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluation and Testing Before Initiating TAGRISSO
TAGRISSO Monotherapy
- •
- Before initiating TAGRISSO monotherapy in patients with cardiac risk factors, conduct cardiac monitoring, including assessment of left ventricular ejection fraction (LVEF) [see Warnings and Precautions (5.3)].
- •
- Before initiating TAGRISSO, perform complete blood count with differential [see Warnings and Precautions (5.7)].
TAGRISSO in Combination with Pemetrexed and Platinum-based Chemotherapy
- •
- Before initiating TAGRISSO in combination with pemetrexed and platinum-based chemotherapy, conduct cardiac monitoring in all patients, including assessment of left ventricular ejection fraction (LVEF) [see Warnings and Precautions (5.3)].
- •
- Before initiating TAGRISSO, perform complete blood count with differential [see Warnings and Precautions (5.7)].
2.2 Patient Selection
Adjuvant Treatment of EGFR Mutation-Positive NSCLC
Select patients with resectable tumors for the adjuvant treatment of NSCLC with TAGRISSO based on the presence of EGFR exon 19 deletions or exon 21 L858R mutations in tumor specimens [see Clinical Studies (14.1)].
First-line Treatment of EGFR Mutation-Positive Metastatic NSCLC
Select patients for the first-line treatment of locally advanced or metastatic EGFR-positive NSCLC with TAGRISSO based on the presence of EGFR exon 19 deletions or exon 21 L858R mutations in tumor or plasma specimens [see Clinical Studies (14.2)]. If these mutations are not detected in a plasma specimen, test tumor tissue if feasible.
First-line Treatment of EGFR Mutation-Positive Locally Advanced or Metastatic NSCLC
Select patients for the first-line treatment of locally advanced or metastatic EGFR-positive NSCLC with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy, based on the presence of EGFR exon 19 deletions or exon 21 L858R mutations in tumor or plasma specimens [see Clinical Studies (14.3)]. If these mutations are not detected in a plasma specimen, test tumor tissue if feasible.
Previously Treated EGFR T790M Mutation-Positive Metastatic NSCLC
Select patients for the treatment of metastatic EGFR T790M mutation-positive NSCLC with TAGRISSO following progression on or after EGFR TKI therapy based on the presence of an EGFR T790M mutation in tumor or plasma specimens [see Clinical Studies (14.4)]. Testing for the presence of the T790M mutation in plasma specimens is recommended only in patients for whom a tumor biopsy cannot be obtained. If this mutation is not detected in a plasma specimen, re-evaluate the feasibility of biopsy for tumor tissue testing.
Information on FDA-approved tests for the detection of EGFR mutations is available at http://www.fda.gov/companiondiagnostics.
2.3 Recommended Dosage and Administration
Recommended Dosage
Table 1 provides the recommended dosage of TAGRISSO by indication.
|
Indication |
Recommended Dosage of TAGRISSO |
Duration of Treatment |
|
Adjuvant Treatment of EGFR Mutation-Positive NSCLC |
80 mg tablet orally once daily with or without food |
For a total of 3 years or until disease recurrence or unacceptable toxicity |
|
First-line Treatment of EGFR Mutation-Positive Metastatic NSCLC |
80 mg tablet orally once daily with or without food |
Until disease progression or unacceptable toxicity |
|
First-line Treatment of EGFR Mutation-Positive Locally Advanced or Metastatic NSCLC |
80 mg tablet orally once daily with or without food in combination with pemetrexed and platinum-based chemotherapy Refer to the Prescribing Information for pemetrexed and cisplatin or carboplatin for the respective dosing information. |
Until disease progression or unacceptable toxicity due to TAGRISSO |
|
Previously Treated EGFR T790M Mutation-Positive Metastatic NSCLC |
80 mg tablet orally once daily with or without food |
Until disease progression or unacceptable toxicity |
Administration
Administer TAGRISSO 80 mg tablet orally once daily with or without food. Tablets may be dispersed in water for patients who have difficulty swallowing, or for nasogastric tube administration [see Dosage and Administration (2.4)].
Missed Dose
If a dose of TAGRISSO is missed, do not make up the missed dose and take the next dose as scheduled.
2.4 Administration to Patients Who Have Difficulty Swallowing Solids
Disperse tablet in 60 mL (2 ounces) of non-carbonated water only. Stir until tablet is dispersed into small pieces (the tablet will not completely dissolve) and swallow immediately. Do not crush, heat, or ultrasonicate during preparation. Rinse the container with 120 mL to 240 mL (4 to 8 ounces) of water and immediately drink.
If administration via nasogastric tube is required, disperse the tablet as above in 15 mL of non-carbonated water, and then use an additional 15 mL of water to transfer any residues to the syringe. The resulting 30 mL liquid should be administered as per the nasogastric tube instructions with appropriate water flushes (approximately 30 mL). Repeat this step until no pieces remain in the syringe. This will help to ensure that the full prescribed dose of the TAGRISSO is given. The dispersion and residues should be administered within 30 minutes of the addition of the tablets to water.
2.5 Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions are provided in Table 2.
|
Target Organ |
Adverse Reaction* |
Dosage Modification |
|
Pulmonary [see Warnings and Precautions (5.1)] |
Interstitial lung disease (ILD)/Pneumonitis |
Permanently discontinue TAGRISSO. |
|
QTc† interval greater than 500 msec on at least 2 separate ECGs‡ |
Withhold TAGRISSO until QTc interval is less than 481 msec or recovery to baseline if baseline QTc is greater than or equal to 481 msec, then resume at 40 mg dose. |
|
|
QTc interval prolongation with signs/symptoms of life-threatening arrhythmia |
Permanently discontinue TAGRISSO. |
|
|
Symptomatic congestive heart failure |
Permanently discontinue TAGRISSO. |
|
|
Cutaneous [see Warnings and Precautions (5.5)] |
Erythema Multiforme Major (EMM), Stevens-Johnson syndrome (SJS), and Toxic Epidermal Necrolysis (TEN) |
Withhold TAGRISSO if suspected and permanently discontinue if confirmed. |
|
Blood and bone marrow [see Warnings and Precautions (5.7)] |
Aplastic anemia |
Withhold TAGRISSO if aplastic anemia is suspected and permanently discontinue if confirmed. |
|
Other [see Adverse Reactions (6.1)] |
Adverse reaction of Grade 3 or greater severity |
Withhold TAGRISSO for up to 3 weeks. |
|
If improvement to Grade 0-2 within 3 weeks |
Resume at 80 mg or 40 mg daily. |
|
|
If no improvement within 3 weeks |
Permanently discontinue TAGRISSO. |
|
Dosage Modifications for Combination Therapy
When TAGRISSO is administered in combination with pemetrexed and platinum-based chemotherapy, modify the dose of any one of the treatments for the management of adverse reactions, as appropriate. For TAGRISSO dose modification instructions, see Table 2. Withhold, reduce the dose or permanently discontinue pemetrexed, cisplatin or carboplatin according to their respective Prescribing Information.
Drug Interactions
Strong CYP3A4 Inducers
Avoid concomitant use of strong CYP3A4 inducers with TAGRISSO. If concurrent use is unavoidable, increase TAGRISSO dosage to 160 mg daily when co-administering with a strong CYP3A inducer. Resume TAGRISSO at 80 mg 3 weeks after discontinuation of the strong CYP3A4 inducer [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
80 mg tablets: beige, oval and biconvex tablet marked with “AZ 80” on one side and plain on the reverse.
40 mg tablets: beige, round and biconvex tablet marked with “AZ 40” on one side and plain on the reverse.
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease/Pneumonitis
Interstitial lung disease (ILD)/pneumonitis occurred in 4% of the 1813 TAGRISSO-treated patients; 0.4% of cases were fatal.
In the FLAURA2 study, ILD/pneumonitis occurred in 3.3% of the 276 patients who received TAGRISSO in combination with pemetrexed and platinum-based chemotherapy; 0.4% of cases were fatal.
Withhold TAGRISSO and promptly investigate for ILD in patients who present with worsening of respiratory symptoms which may be indicative of ILD (e.g., dyspnea, cough and fever). Permanently discontinue TAGRISSO if ILD/pneumonitis is confirmed [see Dosage and Administration (2.5) and Adverse Reactions (6.1)].
5.2 QTc Interval Prolongation
Heart rate-corrected QT (QTc) interval prolongation occurs in patients treated with TAGRISSO. Of the 1813 patients treated with TAGRISSO monotherapy in clinical trials, 1.1% were found to have a QTc >500 msec, and 4.3% of patients had an increase from baseline QTc >60 msec [see Clinical Pharmacology (12.2)].
Of the 276 patients treated with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy in the FLAURA2 study, 1.8% were found to have a QTc >500 msec, and 10.5% of patients had an increase from baseline QTc >60 msec.
No QTc-related arrhythmias were reported.
Clinical trials of TAGRISSO did not enroll patients with baseline QTc of >470 msec. Conduct periodic monitoring with ECGs and electrolytes in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval. Permanently discontinue TAGRISSO in patients who develop QTc interval prolongation with signs/symptoms of life-threatening arrhythmia [see Dosage and Administration (2.5)].
5.3 Cardiomyopathy
Across clinical trials, cardiomyopathy (defined as cardiac failure, chronic cardiac failure, congestive heart failure, pulmonary edema or decreased ejection fraction) occurred in 3.8% of the 1813 TAGRISSO-treated patients; 0.1% of cardiomyopathy cases were fatal.
In the FLAURA2 study, cardiomyopathy occurred in 9% of the 276 patients who received TAGRISSO in combination with pemetrexed and platinum-based chemotherapy; 1.1% of cardiomyopathy cases were fatal.
A decline in left ventricular ejection fraction (LVEF) ≥10 percentage points from baseline and to less than 50% LVEF occurred in 4.2% of 1557 patients who had baseline and at least one follow-up LVEF assessment. In the ADAURA study, 1.5% (5/325) of patients treated with TAGRISSO experienced LVEF decreases greater than or equal to 10 percentage points and a drop to less than 50%. In the FLAURA2 study, 8% (21/262) of patients treated with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy, who had baseline and at least one follow-up LVEF assessment, experienced LVEF decreases greater than or equal to 10 percentage points and a drop to less than 50%.
For patients who will be receiving TAGRISSO monotherapy, conduct cardiac monitoring, including assessment of LVEF at baseline and during treatment, in patients with cardiac risk factors.
For patients who will be receiving TAGRISSO in combination with pemetrexed and platinum-based chemotherapy, conduct cardiac monitoring, including assessment of LVEF at baseline and during treatment, in all patients.
Assess LVEF in patients who develop relevant cardiac signs or symptoms during treatment. For symptomatic congestive heart failure, permanently discontinue TAGRISSO [see Dosage and Administration (2.5)].
5.4 Keratitis
Keratitis was reported in 0.6% of 1813 patients treated with TAGRISSO monotherapy in clinical trials. Promptly refer patients with signs and symptoms suggestive of keratitis (such as eye inflammation, lacrimation, light sensitivity, blurred vision, eye pain and/or red eye) to an ophthalmologist.
5.5 Erythema Multiforme Major, Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis
Postmarketing cases consistent with erythema multiforme major (EMM), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in patients receiving TAGRISSO [see Postmarketing (6.2)]. Withhold TAGRISSO if EMM, SJS, or TEN is suspected and permanently discontinue if confirmed.
5.6 Cutaneous Vasculitis
Postmarketing cases of cutaneous vasculitis including leukocytoclastic vasculitis, urticarial vasculitis, and IgA vasculitis have been reported in patients receiving TAGRISSO [see Postmarketing (6.2)]. Withhold TAGRISSO if cutaneous vasculitis is suspected, evaluate for systemic involvement, and consider dermatology consultation. If no other etiology can be identified, consider permanent discontinuation of TAGRISSO based on severity.
5.7 Aplastic Anemia
Aplastic anemia has been reported in patients treated with TAGRISSO in clinical trials (0.06% of 1813) and postmarketing [see Postmarketing (6.2)]. Some cases had a fatal outcome. Inform patients of the signs and symptoms of aplastic anemia including but not limited to, new or persistent fevers, bruising, bleeding, and pallor. If aplastic anemia is suspected, withhold TAGRISSO and obtain a hematology consultation. If aplastic anemia is confirmed, permanently discontinue TAGRISSO [see Dosage and Administration (2.5)].
Perform complete blood count with differential before starting TAGRISSO, periodically throughout treatment, and more frequently if indicated.
5.8 Embryo-Fetal Toxicity
Based on data from animal studies and its mechanism of action, TAGRISSO can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, osimertinib caused post-implantation fetal loss when administered during early development at a dose exposure 1.5 times the exposure at the recommended clinical dose. When males were treated prior to mating with untreated females, there was an increase in preimplantation embryonic loss at plasma exposures of approximately 0.5 times those observed at the recommended dose of 80 mg once daily. Verify pregnancy status of females of reproductive potential prior to initiating TAGRISSO. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TAGRISSO and for 6 weeks after the final dose. Advise males with female partners of reproductive potential to use effective contraception for 4 months after the final dose [see Use in Specific Populations (8.1and 8.3)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.1)]
- •
- QTc Interval Prolongation [see Warnings and Precautions (5.2)]
- •
- Cardiomyopathy [see Warnings and Precautions (5.3)]
- •
- Keratitis [see Warnings and Precautions (5.4)]
- •
- Erythema multiforme, Stevens-Johnson syndrome, and Toxic epidermal necrolysis [see Warnings and Precautions (5.5)]
- •
- Cutaneous Vasculitis [see Warnings and Precautions (5.6)]
- •
- Aplastic Anemia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS section reflect exposure to TAGRISSO in 1813 patients with EGFR mutation-positive NSCLC who received TAGRISSO monotherapy at the recommended dose of 80 mg once daily in four randomized, controlled trials [ADAURA (n=337), FLAURA (n=338), FLAURA2 (monotherapy arm; n=275), and AURA3 (n=279)], two single arm trials [AURA Extension (n=201) and AURA2 (n=210)], and one dose-finding study, AURA1 (n=173) [see WARNINGS AND PRECAUTIONS (5)]. Among 1813 patients who received TAGRISSO monotherapy, 82% were exposed for 6 months or longer and 67% were exposed for greater than one year. In this pooled safety population, the most common adverse reactions in ≥20% of 1813 patients who received TAGRISSO monotherapy were diarrhea (47%), rash (46%), musculoskeletal pain (38%), nail toxicity (34%), dry skin (32%), stomatitis (24%), and fatigue (21%). The most common laboratory abnormalities in ≥20% of 1813 patients who received TAGRISSO monotherapy were leukopenia (65%), lymphopenia (64%), thrombocytopenia (53%), anemia (52%), and neutropenia (36%).
The data described below reflect exposure to TAGRISSO (80 mg daily) in 337 patients with EGFR mutation-positive resectable NSCLC, and 833 patients with EGFR mutation-positive locally advanced or metastatic NSCLC in four randomized, controlled trials [ADAURA (n=337), FLAURA (n=279), FLAURA2 (monotherapy arm; n=275), and AURA3 (n=279)]. The data also reflect exposure to TAGRISSO at the recommended dose of 80 mg daily given in combination with pemetrexed and platinum-based chemotherapy in 276 patients with EGFR mutation-positive locally advanced or metastatic NSCLC in one randomized controlled trial [FLAURA2 (n=276)]. Patients with a history of interstitial lung disease, drug induced interstitial disease or radiation pneumonitis that required steroid treatment, serious arrhythmia or baseline QTc interval greater than 470 msec on electrocardiogram were excluded from enrollment in these studies.
Adjuvant Treatment of EGFR Mutation-Positive NSCLC - Monotherapy
The safety of TAGRISSO was evaluated in ADAURA, a randomized, double-blind, placebo-controlled trial for the adjuvant treatment of patients with EGFR exon 19 deletions or exon 21 L858R mutation-positive NSCLC who had complete tumor resection, with or without prior adjuvant chemotherapy. At time of DFS analysis, the median duration of exposure to TAGRISSO was 22.5 months.
Serious adverse reactions were reported in 16% of patients treated with TAGRISSO. The most common serious adverse reaction (≥1%) was pneumonia (1.5%). Adverse reactions leading to dose reductions occurred in 9% of patients treated with TAGRISSO. The most frequent adverse reactions leading to dose reductions or interruptions were diarrhea (4.5%), stomatitis (3.9%), nail toxicity (1.8%) and rash (1.8%). Adverse reactions leading to permanent discontinuation occurred in 11% of patients treated with TAGRISSO. The most frequent adverse reactions leading to discontinuation of TAGRISSO were interstitial lung disease (2.7%), and rash (1.2%).
Tables 3 and 4 summarize common adverse reactions and laboratory abnormalities which occurred in ADAURA.
| Adverse Reaction | TAGRISSO (N=337) | PLACEBO (N=343) |
||
|---|---|---|---|---|
| All Grades
(%) | Grade 3 or higher†
(%) | All Grades
(%) | Grade 3 or higher†
(%) |
|
|
||||
|
Gastrointestinal Disorders |
||||
|
Diarrhea‡ |
47 |
2.4 |
20 |
0.3 |
|
Stomatitis§ |
32 |
1.8 |
7 |
0 |
|
Abdominal Pain¶ |
12 |
0.3 |
7 |
0 |
|
Skin Disorders |
||||
|
Rash# |
40 |
0.6 |
19 |
0 |
|
Nail toxicityÞ |
37 |
0.9 |
3.8 |
0 |
|
Dry skinß |
29 |
0.3 |
7 |
0 |
|
Pruritusà |
19 |
0 |
9 |
0 |
|
Respiratory, Thoracic and Mediastinal Disorders |
||||
|
Coughè |
19 |
0 |
19 |
0 |
|
Musculoskeletal and Connective Tissue Disorders |
||||
|
Musculoskeletal Painð |
18 |
0.3 |
25 |
0.3 |
|
Infection and Infestation Disorders |
||||
|
Nasopharyngitis |
14 |
0 |
10 |
0 |
|
Upper respiratory tract infection |
13 |
0.6 |
10 |
0 |
|
Urinary Tract Infectionø |
10 |
0.3 |
7 |
0 |
|
General Disorders and Administration Site Conditions |
||||
|
Fatigueý |
13 |
0.6 |
9 |
0.3 |
|
Nervous System Disorders | ||||
|
Dizziness£ |
10 |
0 |
9 |
0 |
|
Metabolism and Nutrition Disorders |
||||
|
Decreased appetite |
13 |
0.6 |
3.8 |
0 |
Clinically relevant adverse reactions in ADAURA in <10% of patients receiving TAGRISSO were alopecia (6%), epistaxis (6%), interstitial lung disease (3%), palmar-plantar erythrodysesthesia syndrome (1.8%), skin hyperpigmentation (1.8%), urticaria (1.5%), keratitis (0.6%), QTc interval prolongation (0.6%), and erythema multiforme (0.3%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500 msec.
| Laboratory Abnormality*† | TAGRISSO
(N=337) | PLACEBO
(N=343) |
|||
|---|---|---|---|---|---|
| All Grades (%) | Grade 3 or Grade 4 (%) | All Grades
(%) | Grade 3 or Grade 4
(%) |
||
|
Hematology |
|||||
|
Leukopenia |
54 |
0 |
25 |
0 |
|
|
Thrombocytopenia |
47 |
0 |
7 |
0.3 |
|
|
Lymphopenia |
44 |
3.4 |
14 |
0.9 |
|
|
Anemia |
30 |
0 |
12 |
0.3 |
|
|
Neutropenia |
26 |
0.6 |
10 |
0.3 |
|
|
Chemistry |
|||||
|
Hyperglycemia |
25 |
2.3 |
30 |
0.9 |
|
|
Hypermagnesemia |
24 |
1.3 |
14 |
1.5 |
|
|
Hyponatremia |
20 |
1.8 |
16 |
1.5 |
|
Laboratory abnormalities in ADAURA that occurred in <20% of patients receiving TAGRISSO was increased blood creatinine (10%).
Previously Untreated EGFR Mutation-Positive Metastatic Non-Small Cell Lung Cancer - Monotherapy
The safety of TAGRISSO was evaluated in FLAURA, a multicenter international double-blind randomized (1:1) active‑controlled trial conducted in 556 patients with EGFR exon 19 deletion or exon 21 L858R mutation-positive, unresectable or metastatic NSCLC who had not received previous systemic treatment for advanced disease. The median duration of exposure to TAGRISSO was 16.2 months.
Serious adverse reactions were reported in 4% of patients treated with TAGRISSO; the most common serious adverse reactions (≥1%) were pneumonia (2.9%), ILD/pneumonitis (2.1%), and pulmonary embolism (1.8%). Dose reductions occurred in 2.9% of patients treated with TAGRISSO. The most frequent adverse reactions leading to dose reductions or interruptions were prolongation of the QT interval as assessed by ECG (4.3%), diarrhea (2.5%), and lymphopenia (1.1%). Adverse reactions leading to permanent discontinuation occurred in 13% of patients treated with TAGRISSO. The most frequent adverse reaction leading to discontinuation of TAGRISSO was ILD/pneumonitis (3.9%).
Tables 5 and 6 summarize common adverse reactions and laboratory abnormalities which occurred in FLAURA.
|
||||
|
Adverse Reaction |
TAGRISSO (N=279) |
EGFR TKI comparator (gefitinib or erlotinib) (N=277) |
||
|
Any Grade (%) |
Grade 3 or higher (%) |
Any Grade (%) |
Grade 3 or higher (%) |
|
|
Gastrointestinal Disorders |
||||
|
Diarrhea† |
58 |
2.2 |
57 |
2.5 |
|
Stomatitis‡ |
32 |
0.7 |
22 |
1.1 |
|
Nausea |
14 |
0 |
19 |
0 |
|
Constipation |
15 |
0 |
13 |
0 |
|
Vomiting |
11 |
0 |
11 |
1.4 |
|
Skin Disorders |
||||
|
Rash§ |
58 |
1.1 |
78 |
7 |
|
Dry skin¶ |
36 |
0.4 |
36 |
1.1 |
|
Nail toxicity# |
35 |
0.4 |
33 |
0.7 |
|
PruritusÞ |
17 |
0.4 |
17 |
0 |
|
General Disorders and Administration Site Conditions |
||||
|
Fatigueß |
21 |
1.4 |
15 |
1.4 |
|
Pyrexia |
10 |
0 |
4 |
0.4 |
|
Metabolism and Nutrition Disorders |
||||
|
Decreased appetite |
20 |
2.5 |
19 |
1.8 |
|
Respiratory, Thoracic and Mediastinal Disorders |
||||
|
Cough |
17 |
0 |
15 |
0.4 |
|
Dyspnea |
13 |
0.4 |
7 |
1.4 |
|
Neurologic Disorders |
||||
|
Headache |
12 |
0.4 |
7 |
0 |
|
Cardiac Disorders |
||||
|
Prolonged QT Intervalà |
10 |
2.2 |
4 |
0.7 |
|
Infection and Infestation Disorders |
||||
|
Upper Respiratory Tract Infection |
10 |
0 |
7 |
0 |
Clinically relevant adverse reactions in FLAURA in <10% of patients receiving TAGRISSO were alopecia (7%), epistaxis (6%), interstitial lung disease (3.9%), urticaria (2.2%), palmar-plantar erythrodysesthesia syndrome (1.4%), QTc interval prolongation (1.1%), keratitis (0.4%), and skin hyperpigmentation (0.4)%. QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500 msec.
|
||||
|
TAGRISSO (N=279) |
EGFR TKI comparator (gefitinib or erlotinib) (N=277) |
|||
|
All Grades (%) |
Grade 3 or Grade 4 (%) |
All Grades (%) |
Grade 3 or Grade 4 (%) |
|
|
Hematology |
||||
|
Lymphopenia |
63 |
6 |
36 |
4.2 |
|
Anemia |
59 |
0.7 |
47 |
0.4 |
|
Thrombocytopenia |
51 |
0.7 |
12 |
0.4 |
|
Neutropenia |
41 |
3 |
10 |
0 |
|
Chemistry |
||||
|
Hyperglycemia‡ |
37 |
0 |
31 |
0.5 |
|
Hypermagnesemia |
30 |
0.7 |
11 |
0.4 |
|
Hyponatremia |
26 |
1.1 |
27 |
1.5 |
|
Increased AST |
22 |
1.1 |
43 |
4.1 |
|
Increased ALT |
21 |
0.7 |
52 |
8 |
|
Hypokalemia |
16 |
0.4 |
22 |
1.1 |
|
Hyperbilirubinemia |
14 |
0 |
29 |
1.1 |
Clinically relevant laboratory abnormalities in FLAURA that occurred in <20% of patients receiving TAGRISSO was increased blood creatinine (9%).
Previously Untreated EGFR Mutation-Positive Locally Advanced or Metastatic Non-Small Cell Lung Cancer – TAGRISSO in Combination with Pemetrexed and Platinum-based Chemotherapy
The safety of TAGRISSO in combination with pemetrexed and platinum-based chemotherapy was evaluated in FLAURA2, a multicenter international open-label, randomized (1:1), active-controlled trial conducted in 557 patients with EGFR exon 19 deletion or exon 21 L858R mutation-positive, locally advanced or metastatic NSCLC who had not received previous systemic treatment for advanced disease. The median duration of exposure to TAGRISSO in combination with pemetrexed and platinum-based chemotherapy was 22.3 months and the median duration of exposure to TAGRISSO monotherapy was 19.3 months.
Serious adverse reactions were reported in 38% of patients treated with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy; the most frequently reported serious adverse reactions (≥2%) in the combination arm were anemia (3.3%), COVID-19 (2.5%), pneumonia (2.5%), febrile neutropenia (2.2%), thrombocytopenia (2.2%), and pulmonary embolism (2.2%). Fatal adverse reactions occurred in 7% of patients who received TAGRISSO in combination with pemetrexed and platinum-based chemotherapy including pulmonary embolism (1.1%), pneumonia (1.1%) and cardiomyopathy (1.1%).
Dosage interruptions of TAGRISSO, when given with pemetrexed and platinum-based chemotherapy, due to an adverse reaction occurred in 44% of patients. Adverse reactions which required dosage interruption in ≥ 2% of patients included anemia (4.7%), neutropenia (4.3%), diarrhea (3.6%), febrile neutropenia (3.3%) and thrombocytopenia (2.9%).
Permanent discontinuation of TAGRISSO when given in combination with pemetrexed and platinum-based chemotherapy due to an adverse reaction occurred in 11% of patients. Adverse reactions which resulted in permanent discontinuation of TAGRISSO in ≥1% of patients included ILD/pneumonitis (2.9%), pneumonia (1.4%), and decreased ejection fraction (1.1%).
Adverse reactions leading to dose reduction of TAGRISSO occurred in 10% of patients treated with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy. The most frequently reported adverse reactions leading to dose reduction of TAGRISSO in the combination arm in ≥1% of patients were diarrhea (1.1%) and rash (1.1%).
Tables 7 and 8 summarize common adverse reactions and laboratory abnormalities which occurred in FLAURA2.
| Adverse Reaction | TAGRISSO with Pemetrexed and Platinum-based Chemotherapy (N=276) | TAGRISSO
(N=275) |
||
|---|---|---|---|---|
| Any Grade (%) | Grade 3 or higher
(%) | Any Grade (%) | Grade 3 or higher
(%) |
|
|
||||
|
Gastrointestinal Disorders |
||||
|
Diarrhea |
43 |
2.9 |
41 |
0.4 |
|
Stomatitis† |
31 |
0.4 |
21 |
0.4 |
|
Skin Disorders |
||||
|
Rash‡ |
49 |
2.5 |
44 |
1.5 |
|
Nail toxicity§ |
27 |
0.7 |
32 |
0.4 |
|
Dry skin¶ |
24 |
0 |
31 |
0 |
|
Pruritus# |
8 |
0 |
11 |
0 |
Clinically relevant adverse reactions in FLAURA2 in <10% of patients receiving TAGRISSO in combination with pemetrexed and platinum-based chemotherapy were alopecia (9%), epistaxis (7%), palmar-plantar erythrodysesthesia syndrome (5%), interstitial lung disease (3.3%), skin hyperpigmentation (2.5%), QTc interval prolongation (1.8%), erythema multiforme (1.4%), urticaria (1.4%), and keratitis (0.7%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500 msec.
| Laboratory Abnormality‡ | TAGRISSO with Pemetrexed and Platinum-based Chemotherapy (N=276) | TAGRISSO
(N=275) |
||
|---|---|---|---|---|
| All Grades (%) | Grade 3 or Grade 4
(%) | All Grades
(%) | Grade 3 or Grade 4
(%) |
|
|
||||
|
Hematology |
||||
|
Leukopenia |
88 |
20 |
53 |
3.3 |
|
Thrombocytopenia |
85 |
16 |
44 |
1.8 |
|
Neutropenia |
85 |
36 |
40 |
4.7 |
|
Lymphopenia |
78 |
16 |
55 |
7 |
|
Chemistry |
||||
|
Blood creatinine increased |
22 |
0.4 |
8 |
0 |
Previously Treated EGFR T790M Mutation-Positive Metastatic Non-Small Cell Lung Cancer – Monotherapy
The safety of TAGRISSO was evaluated in AURA3, a multicenter international open label randomized (2:1) controlled trial conducted in 419 patients with unresectable or metastatic EGFR T790M mutation-positive NSCLC who had progressive disease following first line EGFR TKI treatment. A total of 279 patients received TAGRISSO 80 mg orally once daily until intolerance to therapy, disease progression, or investigator determination that the patient was no longer benefiting from treatment. A total of 136 patients received pemetrexed plus either carboplatin or cisplatin every three weeks for up to 6 cycles; patients without disease progression after 4 cycles of chemotherapy could continue maintenance pemetrexed until disease progression, unacceptable toxicity, or investigator determination that the patient was no longer benefiting from treatment. Left Ventricular Ejection Fraction (LVEF) was evaluated at screening and every 12 weeks. The median duration of treatment was 8.1 months for patients treated with TAGRISSO and 4.2 months for chemotherapy-treated patients. The trial population characteristics were: median age 62 years, age less than 65 (58%), female (64%), Asian (65%), never smokers (68%), and ECOG PS 0 or 1 (100%).
Serious adverse reactions were reported in 18% of patients treated with TAGRISSO and 26% in the chemotherapy group. No single serious adverse reaction was reported in 2% or more patients treated with TAGRISSO. One patient (0.4%) treated with TAGRISSO experienced a fatal adverse reaction (ILD/pneumonitis).
Dose reductions occurred in 2.9% of patients treated with TAGRISSO. The most frequent adverse reactions leading to dose reductions or interruptions were prolongation of the QT interval as assessed by ECG (1.8%), neutropenia (1.1%), and diarrhea (1.1%). Adverse reactions resulting in permanent discontinuation of TAGRISSO occurred in 7% of patients treated with TAGRISSO. The most frequent adverse reaction leading to discontinuation of TAGRISSO was ILD/pneumonitis (3%).
Tables 9 and 10 summarize common adverse reactions and laboratory abnormalities which occurred in TAGRISSO-treated patients in AURA3.
|
|||||
|
Adverse Reaction |
TAGRISSO (N=279) |
Chemotherapy (Pemetrexed/Cisplatin or Pemetrexed/Carboplatin) (N=136) |
|||
|
All Grades† (%) |
Grade 3/4† (%) |
All Grades† (%) |
Grade 3/4† (%) |
||
|
Gastrointestinal Disorders |
|||||
|
Diarrhea |
41 |
1.1 |
11 |
1.5 |
|
|
Nausea |
16 |
0.7 |
49 |
3.7 |
|
|
Stomatitis‡ |
19 |
0 |
15 |
1.5 |
|
|
Constipation |
14 |
0 |
35 |
0 |
|
|
Vomiting |
11 |
0.4 |
20 |
2.2 |
|
|
Skin Disorders |
|||||
|
Rash§ |
34 |
0.7 |
6 |
0 |
|
|
Dry skin¶ |
23 |
0 |
4.4 |
0 |
|
|
Nail toxicity# |
22 |
0 |
1.5 |
0 |
|
|
PruritusÞ |
13 |
0 |
5 |
0 |
|
|
General Disorders and Administration Site Conditions |
|||||
|
Fatigueß |
22 |
1.8 |
40 |
5.1 |
|
|
Metabolism and Nutrition Disorders |
|||||
|
Decreased appetite |
18 |
1.1 |
36 |
2.9 |
|
|
Respiratory, Thoracic and Mediastinal Disorders |
|||||
|
Cough |
17 |
0 |
14 |
0 |
|
|
Musculoskeletal and Connective Tissue Disorders |
|||||
|
Back pain |
10 |
0.4 |
9 |
0.7 |
|
Clinically relevant adverse reactions in AURA3 in <10% of patients receiving TAGRISSO were epistaxis (5%), interstitial lung disease (3.9%), alopecia (3.6%), urticaria (2.9%), palmar-plantar erythrodysesthesia syndrome (1.8%), QTc interval prolongation (1.4%), keratitis (1.1%), and erythema multiforme (0.7%), and skin hyperpigmentation (0.4%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500 msec.
|
|||||
|
TAGRISSO (N=279) |
Chemotherapy (Pemetrexed/Cisplatin or Pemetrexed/Carboplatin) (N=131) |
||||
|
All Grades (%) |
Grade 3 or Grade 4 (%) |
All Grades (%) |
Grade 3 or Grade 4 (%) |
||
|
Hematology |
|||||
|
Anemia |
43 |
0 |
79 |
3.1 |
|
|
Lymphopenia |
63 |
8 |
61 |
10 |
|
|
Thrombocytopenia |
46 |
0.7 |
48 |
7 |
|
|
Neutropenia |
27 |
2.2 |
49 |
12 |
|
|
Chemistry |
|||||
|
Hypermagnesemia† |
27 |
1.8 |
9 |
1.5 |
|
|
Hyponatremia† |
26 |
2.2 |
36 |
1.5 |
|
|
Hyperglycemia‡ |
20 |
0 |
NA |
NA |
|
|
Hypokalemia† |
9 |
1.4 |
18 |
1.5 |
|
|
NA=Not Applicable |
|||||
Clinically relevant laboratory abnormalities in AURA3 that occurred in <20% of patients receiving TAGRISSO included increased blood creatinine (7%).
Other Clinical Trials Experience
The following adverse reaction has been reported following administration of TAGRISSO: increased blood creatine phosphokinase.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TAGRISSO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
- •
- Skin and subcutaneous tissue: Erythema multiforme major (EMM), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), cutaneous vasculitis, erythema dyschromicum perstans
- •
- Blood and lymphatic system disorders: Aplastic anemia
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Osimertinib
Strong CYP3A Inducers
Co-administering TAGRISSO with a strong CYP3A4 inducer decreased the exposure of osimertinib compared to administering TAGRISSO alone [see Clinical Pharmacology (12.3)]. Decreased osimertinib exposure may lead to reduced efficacy.
Avoid co-administering TAGRISSO with strong CYP3A inducers. Increase the TAGRISSO dosage when co-administering with a strong CYP3A4 inducer if concurrent use is unavoidable [see Dosage and Administration (2.5)]. No dose adjustments are required when TAGRISSO is used with moderate and/or weak CYP3A inducers.
7.2 Effect of Osimertinib on Other Drugs
Co-administering TAGRISSO with a breast cancer resistant protein (BCRP) or P-glycoprotein (P-gp) substrate increased the exposure of the substrate compared to administering it alone [see Clinical Pharmacology (12.3)]. Increased BCRP or P-gp substrate exposure may increase the risk of exposure-related toxicity.
Monitor for adverse reactions of the BCRP or P-gp substrate, unless otherwise instructed in its approved labeling, when co-administered with TAGRISSO.
7.3 Drugs That Prolong the QTc Interval
The effect of co-administering medicinal products known to prolong the QTc interval with TAGRISSO is unknown. When feasible, avoid concomitant administration of drugs known to prolong the QTc interval with known risk of Torsades de pointes. If not feasible to avoid concomitant administration of such drugs, conduct periodic ECG monitoring [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on data from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], TAGRISSO can cause fetal harm when administered to a pregnant woman. There are no available data on TAGRISSO use in pregnant women. Administration of osimertinib to pregnant rats was associated with embryolethality and reduced fetal growth at plasma exposures 1.5 times the exposure at the recommended clinical dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
When administered to pregnant rats prior to embryonic implantation through the end of organogenesis (gestation days 2-20) at a dose of 20 mg/kg/day, which produced plasma exposures of approximately 1.5 times the clinical exposure, osimertinib caused post-implantation loss and early embryonic death. When administered to pregnant rats from implantation through the closure of the hard palate (gestation days 6 to 16) at doses of 1 mg/kg/day and above (0.1 times the AUC observed at the recommended clinical dose of 80 mg once daily), an equivocal increase in the rate of fetal malformations and variations was observed in treated litters relative to those of concurrent controls. When administered to pregnant dams at doses of 30 mg/kg/day during organogenesis through lactation Day 6, osimertinib caused an increase in total litter loss and postnatal death. At a dose of 20 mg/kg/day, osimertinib administration during the same period resulted in increased postnatal death as well as a slight reduction in mean pup weight at birth that increased in magnitude between lactation days 4 and 6.
8.2 Lactation
Risk Summary
There are no data on the presence of osimertinib or its active metabolites in human milk, the effects of osimertinib on the breastfed infant or on milk production. Administration to rats during gestation and early lactation was associated with adverse effects, including reduced growth rates and neonatal death [see Use in Specific Populations (8.1)]. Because of the potential for serious adverse reactions in breastfed infants from osimertinib, advise women not to breastfeed during treatment with TAGRISSO and for 2 weeks after the final dose.
8.3 Females and Males of Reproductive Potential
Based on animal data, TAGRISSO can cause malformations, embryo lethality, and postnatal death at doses resulting in exposures 1.5 times or less the human exposure at the clinical dose of 80 mg daily [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating TAGRISSO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with TAGRISSO and for 6 weeks after the final dose [see Use in Specific Populations (8.1)].
Males
Advise male patients with female partners of reproductive potential to use effective contraception during and for 4 months following the final dose of TAGRISSO [see Nonclinical Toxicology (13.1)].
Infertility
Based on animal studies, TAGRISSO may impair fertility in females and males of reproductive potential. The effects on female fertility showed a trend toward reversibility. It is not known whether the effects on male fertility are reversible [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of TAGRISSO in pediatric patients have not been established.
8.5 Geriatric Use
Monotherapy
Of the 1813 patients with EGFR exon 19 deletion or exon 21 L858R mutation-positive NSCLC who were treated with TAGRISSO monotherapy, 770 patients were ≥65 years and 207 patients were ≥75 years of age [see Adverse Reactions (6.1)]. Exploratory analysis suggests a higher incidence of Grade 3 or higher adverse reactions (43% vs 33%) and more frequent dosage modifications for adverse reactions (34% vs 23%) in patients 65 years or older as compared to those younger than 65 years. No overall differences in safety or effectiveness were observed between patients 65 years or older and younger patients.
TAGRISSO in Combination with Pemetrexed and Platinum-based Chemotherapy
Of the 276 patients with EGFR exon 19 deletion or exon 21 L858R mutation-positive, locally advanced or metastatic NSCLC treated with TAGRISSO in combination with pemetrexed and platinum-based chemotherapy, 104 patients were ≥65 years and 23 patients were ≥75 years of age [see Adverse Reactions (6.1)]. Exploratory analysis suggests a higher incidence of Grade 3 or higher adverse reactions (68% vs 61%) and more frequent dosage modifications for adverse reactions (55% vs 43%) in patients 65 years or older as compared to those younger than 65 years. Clinical studies of TAGRISSO in combination with pemetrexed and platinum-based chemotherapy did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients.
8.6 Renal Impairment
No dose adjustment is recommended in patients with creatinine clearance (CLcr) 15 - 89 mL/min, as estimated by Cockcroft-Gault. There is no recommended dose of TAGRISSO for patients with end-stage renal disease (CLcr <15 mL/min) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is recommended in patients with mild to moderate hepatic impairment (Child-Pugh A and B or total bilirubin ≤ ULN and AST > ULN or total bilirubin 1 to 3 times ULN and any AST). There is no recommended dose for TAGRISSO for patients with severe hepatic impairment (total bilirubin between 3 to 10 times ULN and any AST) [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
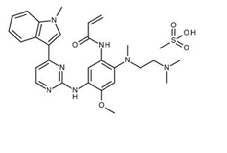
Osimertinib is a kinase inhibitor for oral use. The molecular formula for osimertinib mesylate is C28H33N7O2•CH4O3S, and the molecular weight is 596 g/mol. The chemical name is N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide mesylate salt. Osimertinib has the following structural formula (as osimertinib mesylate):
TAGRISSO tablets contain 40 or 80 mg of osimertinib, equivalent to 47.7 and 95.4 mg of osimertinib mesylate, respectively. Inactive ingredients in the tablet core are mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose and sodium stearyl fumarate. The tablet coating consists of polyvinyl alcohol, titanium dioxide, macrogol 3350, talc, ferric oxide yellow, ferric oxide red and ferric oxide black.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Osimertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR), which binds irreversibly to certain mutant forms of EGFR (T790M, L858R, and exon 19 deletions) at approximately 9-fold lower concentrations than wild-type. Two pharmacologically-active metabolites (AZ7550 and AZ5104 circulating at approximately 10% of the parent) with similar inhibitory profiles to osimertinib have been identified in the plasma after oral administration of osimertinib. AZ7550 showed a similar potency to osimertinib, while AZ5104 showed greater potency against exon 19 deletion and T790M mutants (approximately 8-fold) and wild-type (approximately 15-fold) EGFR. In vitro, osimertinib also inhibited the activity of HER2, HER3, HER4, ACK1, and BLK at clinically relevant concentrations.
In cultured cells and animal tumor implantation models, osimertinib exhibited anti-tumor activity against NSCLC lines harboring EGFR-mutations (T790M/L858R, L858R, T790M/exon 19 deletion, and exon 19 deletion) and, to a lesser extent, wild-type EGFR amplifications. Osimertinib distributed to the brain in multiple animal species (monkey, rat, and mouse) with brain to plasma AUC ratios of approximately 2 following oral dosing. These data are consistent with observations of tumor regression and increased survival in osimertinib- versus control-treated animals in a pre-clinical mutant-EGFR intracranial mouse metastasis xenograft model (PC9; exon 19 deletion).
12.2 Pharmacodynamics
Based on an analysis of dose-exposure response relationships over the dose range of 20 mg (0.25 times the recommended dose) to 240 mg (3 times the recommended dose), no apparent relationship between osimertinib exposure and overall response rate, duration of response and progression-free survival was identified; however, there were limited data available at the 20 mg dose. Over the same dose range, increased exposure led to increased probability of adverse reactions, specifically rash, diarrhea and ILD.
Cardiac Electrophysiology
The QTc interval prolongation potential of osimertinib was assessed in 210 patients who received TAGRISSO 80 mg daily in AURA2. A central tendency analysis of the QTcF data at steady-state demonstrated that the maximum mean change from baseline was 16.2 msec (upper bound of two-sided 90% confidence interval (CI) 17.6 msec). A pharmacokinetic/pharmacodynamic analysis in AURA2 suggested a concentration-dependent QTc interval prolongation of 14 msec (upper bound of two-sided 90% CI: 16 msec) at a dose of TAGRISSO 80 mg.
12.3 Pharmacokinetics
The area under the plasma concentration-time curve (AUC) and maximal plasma concentration (Cmax) of osimertinib increased dose proportionally over 20 to 240 mg dose range (i.e., 0.25 to 3 times the recommended dosage) after oral administration and exhibited linear pharmacokinetics (PK). Administration of TAGRISSO orally once daily resulted in approximately 3-fold accumulation with steady-state exposures achieved after 15 days of dosing. At steady state, the Cmax to Cmin (minimal concentration) ratio was 1.6-fold.
The pharmacokinetics in patients treated with osimertinib in combination with pemetrexed and platinum-based chemotherapy are similar to those in patients treated with osimertinib monotherapy.
Absorption
The median time to Cmax of osimertinib was 6 hours (range 3-24 hours).
Following administration of a 20 mg TAGRISSO tablet with a high-fat, high-calorie meal (containing approximately 58 grams of fat and 1000 calories), the Cmax and AUC of osimertinib were comparable to that under fasting conditions.
Distribution
The mean volume of distribution at steady-state (Vss/F) of osimertinib was 918 L. Plasma protein binding of osimertinib was 95%. PET brain imaging studies in healthy volunteers and in patients with brain metastases show that osimertinib is distributed to the brain following intravenous injection of a micro dose of 11C-labeled osimertinib.
Elimination
Osimertinib plasma concentrations decreased with time and a population estimated mean half-life of osimertinib was 48 hours, and oral clearance (CL/F) was 14.3 (L/h).
Metabolism
The main metabolic pathways of osimertinib were oxidation (predominantly CYP3A) and dealkylation in vitro. Two pharmacologically active metabolites (AZ7550 and AZ5104) have been identified in the plasma after TAGRISSO oral administration. The geometric mean exposure (AUC) of each metabolite (AZ5104 and AZ7550) was approximately 10% of the exposure of osimertinib at steady-state.
Excretion
Osimertinib is primarily eliminated in the feces (68%) and to a lesser extent in the urine (14%). Unchanged osimertinib accounted for approximately 2% of the elimination.
Specific Populations
No clinically significant differences in the pharmacokinetics of osimertinib were observed based on age, sex, ethnicity, body weight, baseline albumin, line of therapy, smoking status, renal function (creatinine clearance (CLcr) ≥15 mL/min by Cockcroft-Gault), or hepatic impairment (Child-Pugh A and B, or total bilirubin ≤ ULN and AST > ULN or total bilirubin between 1 to 3 times ULN and any AST). The pharmacokinetics of osimertinib in patients with end-stage renal disease (CLcr <15 mL/min) or severe hepatic impairment (total bilirubin 3 to 10 times ULN and any AST) are unknown [see Use in Specific Populations (8.6) and (8.7)].
Drug Interaction Studies
Effect of Other Drugs on TAGRISSO in Clinical Pharmacokinetic Studies Strong CYP3A Inducers: The steady-state AUC of osimertinib was reduced by 78% in patients when co-administered with rifampin (600 mg daily for 21 days) [see Drug Interactions (7.1)].
Strong CYP3A Inhibitors: Co-administering TAGRISSO with 200 mg itraconazole twice daily (a strong CYP3A4 inhibitor) had no clinically significant effect on the exposure of osimertinib (AUC increased by 24% and Cmax decreased by 20%).
Gastric Acid Reducing Agents: The exposure of osimertinib was not affected by concurrent administration of a single 80 mg TAGRISSO tablet following 40 mg omeprazole administration for 5 days.
Effect of Osimertinib on Other Drugs in Clinical Pharmacokinetic Studies
BCRP substrates: Co-administering TAGRISSO with rosuvastatin (a BCRP substrate) increased rosuvastatin AUC by 35% and Cmax by 72% [see Drug Interactions (7.2)].
P-gp substrates: Co-administering TAGRISSO with fexofenadine (a P-gp substrate) increased fexofenadine AUC and Cmax by 56% and 76% after a single dose and 27% and 25% at steady state, respectively.
CYP3A4 substrates: Co-administering TAGRISSO with simvastatin (a CYP3A4 substrate) had no clinically significant effect on the exposure of simvastatin.
In Vitro Studies
CYP450 Metabolic Pathways: Osimertinib does not inhibit CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6 and 2E1. Osimertinib induced CYP1A2 enzymes.
Transporter Systems: Osimertinib is a substrate of P-glycoprotein and BCRP and is not a substrate of OATP1B1 and OATP1B3. Osimertinib is an inhibitor of BCRP and does not inhibit OAT1, OAT3, OATP1B1, OATP1B3, MATE1, MATE2K and OCT2.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year carcinogenicity was conducted in male and female rats at oral osimertinib doses of 1, 3, and 10 mg/kg/day. Osimertinib increased the incidences of hemangioma and combined hemangioma/hemangiosarcoma in the mesenteric lymph node and whole body at 10 mg/kg/day (0.2 times the human exposure based on AUC at the clinical dose of 80 mg once daily). Administration of osimertinib to male and female rasH2 transgenic mice by oral gavage daily for 26 weeks did not result in an increased incidence of neoplasms at doses up to 10 mg/kg/day.
Osimertinib did not induce mutations in the bacterial reverse mutation (Ames) assay and was not genotoxic in mouse lymphoma cells or in the rat in vivo micronucleus assay.
Based on studies in animals, male fertility may be impaired by treatment with TAGRISSO. Degenerative changes were present in the testes in rats and dogs exposed to osimertinib for 1 month or more with evidence of reversibility in the rat. Following administration of osimertinib to rats for approximately 10 weeks at a dose of 40 mg/kg, at exposures 0.5 times the AUC observed at the recommended clinical dose of 80 mg once daily, there was a reduction in male fertility, demonstrated by increased pre-implantation loss in untreated females mated to treated males.
Based on studies in animals, female fertility may be impaired by treatment with TAGRISSO. In repeat dose toxicity studies, histological evidence of anestrus, corpora lutea degeneration in the ovaries and epithelial thinning in the uterus and vagina were seen in rats exposed to osimertinib for 1 month or more at exposures 0.3 times the AUC observed at the recommended clinical dose of 80 mg once daily. Findings in the ovaries seen following 1 month of dosing exhibited evidence of reversibility. In a female fertility study in rats, administration of osimertinib from 2 weeks prior to mating through Day 8 of gestation at a dose of 20 mg/kg/day (approximately 1.5 times the Cmax at the recommended dose of 80 mg once daily) had no effects on estrous cycling or the number of females becoming pregnant, but caused early embryonic deaths. These findings showed evidence of reversibility when females were mated 1 month after treatment discontinuation.
13.2 Animal Toxicology and/or Pharmacology
Administration of osimertinib resulted in histological findings of lens fiber degeneration in the 2-year rat carcinogenicity study at ≥3 mg/kg/day (exposures 0.2 times the human exposure based on AUC). These findings were consistent with the ophthalmoscopic observation of lens opacities, which were first noted from week 52 and showed a gradual increase in incidence and severity with increased duration of dosing.
14 CLINICAL STUDIES
14.1 Adjuvant Treatment of Early-Stage EGFR Mutation-Positive Non-Small Cell Lung Cancer (NSCLC)
The efficacy of TAGRISSO was demonstrated in a randomized, double-blind, placebo-controlled trial (ADAURA [NCT02511106]) for the adjuvant treatment of patients with EGFR exon 19 deletions or exon 21 L858R mutation-positive NSCLC who had complete tumor resection, with or without prior adjuvant chemotherapy. Eligible patients with resectable tumors (stage IB – IIIA according to American Joint Commission on Cancer [AJCC] 7th edition) were required to have predominantly non-squamous histology and EGFR exon 19 deletions or exon 21 L858R mutations identified prospectively from tumor tissue in a central laboratory by the cobas® EGFR Mutation Test. Patients with clinically significant uncontrolled cardiac disease, prior history of ILD/pneumonitis, or who received treatment with any EGFR kinase inhibitor were not eligible for the study.
Patients were randomized (1:1) to receive TAGRISSO 80 mg orally once daily or placebo following recovery from surgery and standard adjuvant chemotherapy if given. Patients who did not receive adjuvant chemotherapy were randomized within 10 weeks and patients who received adjuvant chemotherapy were randomized within 26 weeks following surgery. Randomization was stratified by mutation type (exon 19 deletions or exon 21 L858R mutations), race (Asian or non-Asian) and pTNM staging (IB or II or IIIA) according to AJCC 7th edition. Treatment was given for 3 years or until disease recurrence, or unacceptable toxicity.
The major efficacy outcome measure was disease-free survival (DFS, defined as reduction in the risk of disease recurrence or death) in patients with stage II – IIIA NSCLC determined by investigator assessment. Additional efficacy outcome measures included DFS in the overall population (patients with stage IB – IIIA NSCLC), and overall survival (OS) in patients with stage II – IIIA NSCLC and in the overall population.
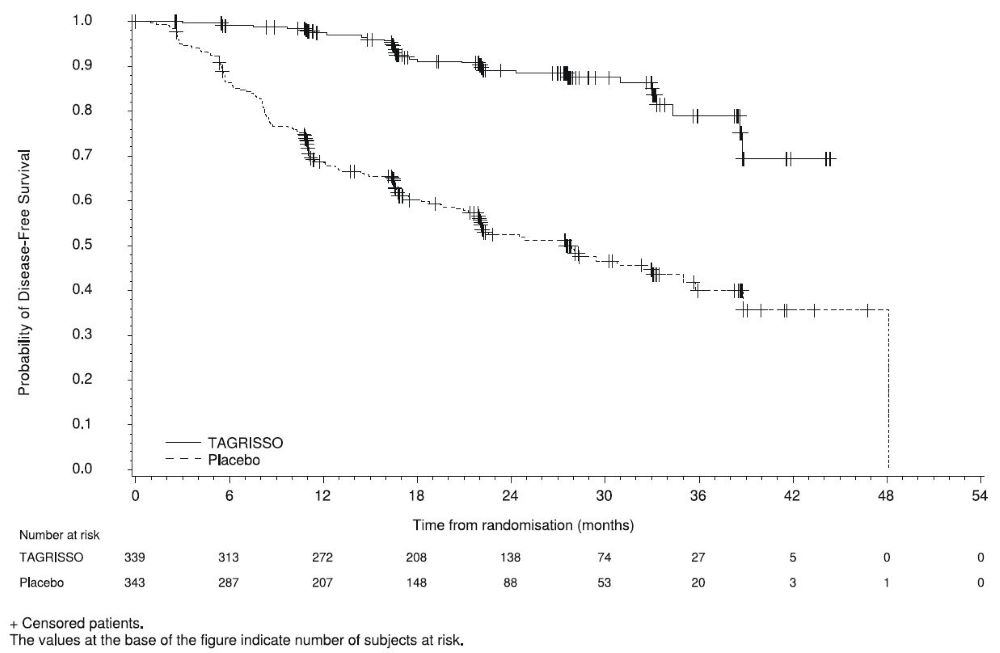
A total of 682 patients were randomized to TAGRISSO (n=339) or placebo (n=343). The median age was 63 years (range 30-86 years); 70% were female; 64% were Asian and 72% were never smokers. Baseline World Health Organization (WHO) performance status was 0 (64%) or 1 (36%); 31% had stage IB, 35% II, and 34% IIIA. With regard to EGFR mutation status, 55% were exon 19 deletions and 45% were exon 21 L858R mutations. The majority (60%) of patients received adjuvant chemotherapy prior to randomization (27% IB; 70% II, 79% IIIA).
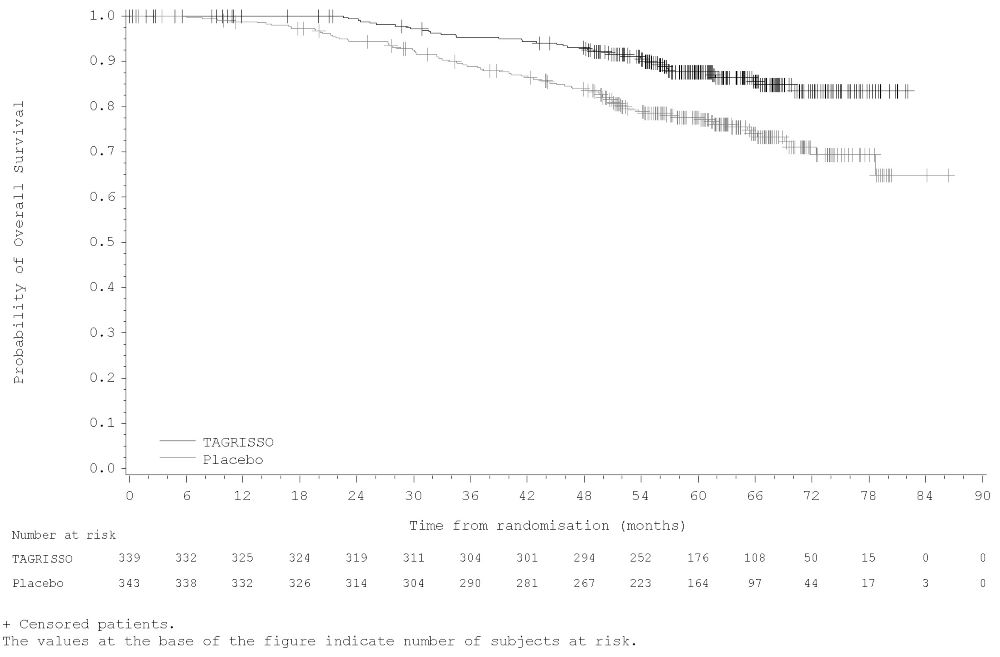
ADAURA demonstrated a statistically significant difference in DFS for patients treated with TAGRISSO compared to patients treated with placebo. The final analysis of OS demonstrated a statistically significant improvement in OS for patients treated with TAGRISSO compared to patients treated with placebo. Median OS was not reached in either arm. In the overall population (IB-IIIA), the median follow-up time was 61.5 months in both treatment arms. Efficacy results from ADAURA are summarized in Table 11 and Figures 1 and 2, respectively.
|
STAGE II-IIIA POPULATION |
STAGE IB-IIIA POPULATION |
|||
|
Efficacy Parameter |
TAGRISSO |
PLACEBO |
TAGRISSO |
PLACEBO |
|
Disease-Free Survival (DFS) |
||||
|
DFS events (%) |
26 (11) |
130 (55) |
37 (11) |
159 (46) |
|
Recurrent disease (%) |
26 (11) |
129 (54) |
37 (11) |
157 (46) |
|
Deaths (%) |
0 |
1 (0.4) |
0 |
2 (0.6) |
|
Median DFS, months (95% CI) |
NR (38.8, NE) |
19.6 (16.6, 24.5) |
NR (NE, NE) |
27.5 (22.0, 35.0) |
|
0.17 (0.12, 0.23) |
0.20 (0.15, 0.27) |
|||
|
<0.0001 |
<0.0001 |
|||
|
Overall Survival (OS) |
||||
|
Number of deaths (%) |
35 (15) |
65 (27) |
42 (12) |
82 (24) |
|
0.49 (0.33, 0.73) |
0.49 (0.34, 0.70) |
|||
|
0.0004 |
<0.0001 |
|||
|
DFS results based on investigator assessment. CI=Confidence Interval; NE=Not Estimable; NR=Not Reached |
||||
Figure 1. Kaplan-Meier Curves of Disease-Free Survival (Overall Population) by Investigator Assessment in ADAURA
Figure 2. Kaplan-Meier Curves of Overall Survival (Overall Population) in ADAURA
In an exploratory analysis of site(s) of relapse, the proportion of patients with CNS involvement at the time of disease recurrence was 5 patients (1.5%) on the TAGRISSO arm and 34 patients (10%) on the placebo arm.
14.2 Previously Untreated EGFR Mutation-Positive Metastatic NSCLC
FLAURA – TAGRISSO Monotherapy
The efficacy of TAGRISSO was demonstrated in a randomized, multicenter, double-blind, active-controlled trial (FLAURA [NCT02296125]) in patients with EGFR exon 19 deletions or exon 21 L858R mutation-positive, metastatic NSCLC, who had not received previous systemic treatment for metastatic disease. Patients were required to have measurable disease per RECIST v1.1, a WHO performance status of 0-1, and EGFR exon 19 deletions or exon 21 L858R mutation in tumor prospectively identified by the cobas® EGFR Mutation Test in a central laboratory or by an investigational assay at a CLIA-certified or accredited laboratory. Patients with CNS metastases not requiring steroids and with stable neurologic status for at least two weeks after completion of definitive surgery or radiotherapy were eligible. Patients were assessed at the investigator’s discretion for CNS metastases if they had a history of, or suspected, CNS metastases at study entry.
Patients were randomized (1:1) to receive TAGRISSO 80 mg orally once daily or to receive gefitinib 250 mg orally once daily or erlotinib 150 mg orally once daily until disease progression or unacceptable toxicity. Randomization was stratified by EGFR mutation type (exon 19 deletions or exon 21 L858R mutation) and ethnicity (Asian or non-Asian). Patients randomized to the control arm were offered TAGRISSO at the time of disease progression if tumor samples tested positive for the EGFR T790M mutation. The major efficacy outcome measure was progression-free survival (PFS), as assessed by investigator. Additional efficacy outcome measures included OS and overall response rate (ORR).
A total of 556 patients were randomized to TAGRISSO (n=279) or to control (gefitinib n=183; erlotinib n=94). The median age was 64 years (range 26-93 years); 54% were <65 years of age; 63% were female; 62% were Asian and 64% were never smokers. Baseline WHO performance status was 0 (41%) or 1 (59%); 5% had Stage IIIb and 95% had Stage IV; and 7% received prior systemic cytotoxic chemotherapy as neoadjuvant or adjuvant therapy. With regard to EGFR tumor testing, 63% were exon 19 deletions and 37% were exon 21 L858R; 5 patients (<1%) also had a concomitant de novo T790M mutation. EGFR mutation status was confirmed centrally using the cobas® EGFR Mutation Test in 90% of patients. At the time of the final data cut-off, of those randomized to TAGRISSO and to investigator’s choice erlotinib or gefitinib arm, 133 (48%) and 180 (65%) patients had received at least one subsequent treatment, respectively. Out of the 180 patients randomized to erlotinib or gefitinib who received subsequent treatment, 85 (47%) patients received TAGRISSO as first subsequent therapy.
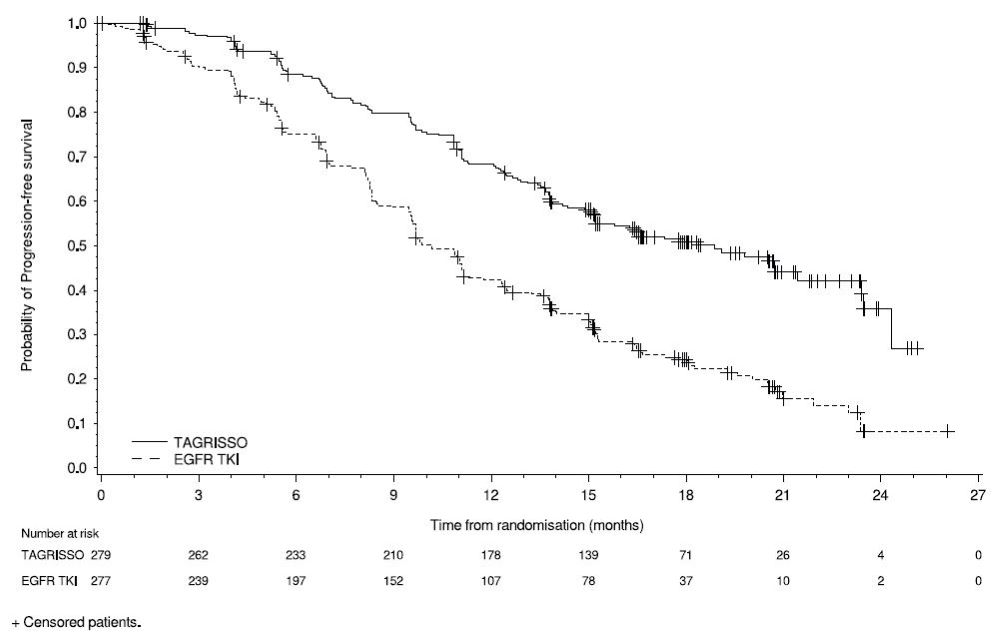
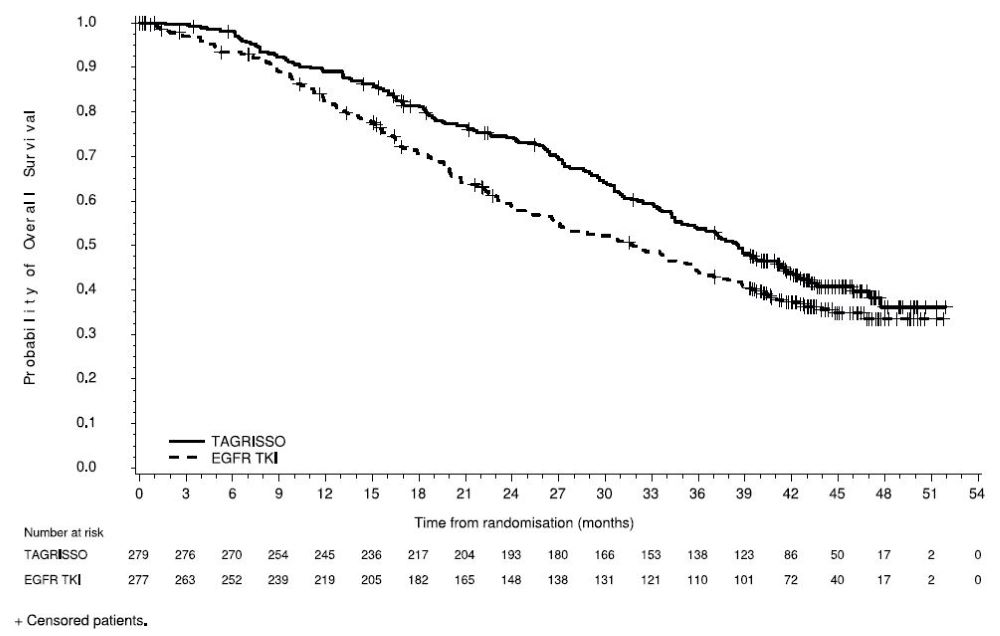
FLAURA demonstrated a statistically significant improvement in PFS for patients randomized to TAGRISSO as compared to erlotinib or gefitinib (see Table 12 and Figure 3). The final analysis of overall survival demonstrated a statistically significant improvement in overall survival in patients randomized to TAGRISSO compared to erlotinib or gefitinib (see Table 12 and Figure 4).
| Efficacy Parameter | TAGRISSO
(N=279) | EGFR TKI
(gefitinib or erlotinib) (N=277) |
|---|---|---|
|
Progression-Free Survival (PFS) |
||
|
PFS events (%) |
136 (49) |
206 (74) |
|
Progressive disease (%) |
125 (45) |
192 (69) |
|
Death* (%) |
11 (4) |
14 (5) |
|
Median PFS in months (95% CI) |
18.9 (15.2, 21.4) |
10.2 (9.6, 11.1) |
|
0.46 (0.37, 0.57) |
||
|
<0.0001 |
||
|
Overall Survival (OS) |
||
|
Number of deaths (%) |
155 (56) |
166 (60) |
|
Median OS in months (95% CI) |
38.6 (34.5, 41.8) |
31.8 (26.6, 36.0) |
|
0.80 (0.64, 1.00) |
||
|
0.0462 |
||
|
Overall Response Rate (ORR)¶ |
||
|
ORR, % (95% CI)† |
77 (71, 82) |
69 (63, 74) |
|
Complete response, % |
2 |
1 |
|
Partial response, % |
75 |
68 |
|
Duration of Response (DoR)¶ |
||
|
Median in months (95% CI) |
17.6 (13.8, 22.0) |
9.6 (8.3, 11.1) |
Figure 3. Kaplan-Meier Curves of PFS by Investigator Assessment in FLAURA
In a supportive analysis of PFS according to blinded independent central review (BICR), median PFS was 17.7 months in the TAGRISSO arm compared to 9.7 months in the EGFR TKI comparator arm (HR=0.45; 95% CI: 0.36, 0.57).
Figure 4. Kaplan-Meier Curves of Overall Survival in FLAURA
Of 556 patients, 200 patients (36%) had baseline brain scans reviewed by BICR; this included 106 patients in the TAGRISSO arm and 94 patients in the investigator choice of EGFR TKI arm. Of these 200 patients, 41 had measurable CNS lesions per RECIST v1.1. Results of pre-specified exploratory analyses of CNS ORR and duration of response (DoR) by BICR in the subset of patients with measurable CNS lesions at baseline are summarized in Table 13.
|
TAGRISSO N=22 |
EGFR TKI (gefitinib or erlotinib) N=19 |
|
|
CNS ORR, % (95% CI) |
77 (55, 92) |
63 (38, 84) |
|
Complete response, % |
18 |
0 |
|
CNS Duration of Response‡ |
||
|
Number of responders |
17 |
12 |
|
Response Duration ≥6 months, % |
88 |
50 |
|
Response Duration ≥12 months, % |
47 |
33 |
14.3 Previously Untreated EGFR Mutation-Positive Locally Advanced or Metastatic NSCLC
FLAURA2 – TAGRISSO in Combination with Pemetrexed and Platinum-based Chemotherapy
The efficacy of TAGRISSO in combination with pemetrexed and platinum-based chemotherapy was demonstrated in a randomized, multicenter, open-label trial (FLAURA2 [NCT04035486]) in patients with EGFR exon 19 deletion or exon 21 L858R mutation-positive locally advanced or metastatic NSCLC, who had not received previous systemic treatment for advanced disease. Patients were required to have measurable disease per RECIST v1.1, a WHO performance status of 0-1, and EGFR exon 19 deletions or exon 21 L858R mutations as identified by the cobas® EGFR Mutation Test v2 performed prospectively in tissue samples in a central laboratory or by a local test performed in a CLIA-certified or accredited laboratory.
Patients were randomized (1:1) to one of the following treatment arms:
- •
- TAGRISSO (80 mg) orally once daily with pemetrexed (500 mg/m2) and investigator’s choice of cisplatin (75 mg/m2) or carboplatin (AUC5) administered intravenously on Day 1 of 21-day cycles for 4 cycles, followed by TAGRISSO (80 mg) orally once daily and pemetrexed (500 mg/m2) administered intravenously every 3 weeks
- •
- TAGRISSO (80 mg) orally once daily
Randomization was stratified by race (Chinese/Asian, non-Chinese/Asian or non-Asian), WHO performance status (0 or 1), and method for tissue testing (central or local). Patients received study therapy until intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit.
Progression-free survival, as assessed by investigator per RECIST 1.1 was the primary efficacy outcome measure. Overall survival was a key secondary outcome measure. Additional efficacy outcome measures included ORR and DoR.
A total of 557 patients were randomized to either TAGRISSO in combination with pemetrexed and platinum-based chemotherapy (n=279) or TAGRISSO monotherapy (n=278). The median age was 61 years (range 26-85 years); 39% were ≥65 years and 8% were ≥75 years of age; 61% were female; 64% were Asian and 66% were never smokers. Baseline WHO PS was 0 (37%) or 1 (63%); 4% had locally advanced and 96% had metastatic NSCLC; and 1.8% received prior systemic cytotoxic chemotherapy as neoadjuvant or adjuvant therapy. With regard to EGFR tumor testing, 61% of tumors had exon 19 deletions and 38% had exon 21 L858R mutations; 0.7% of patients had tumors with both exon 19 deletions and exon 21 L858R. EGFR mutation status was centrally confirmed using the cobas® EGFR Mutation Test v2 in 96% of patients.
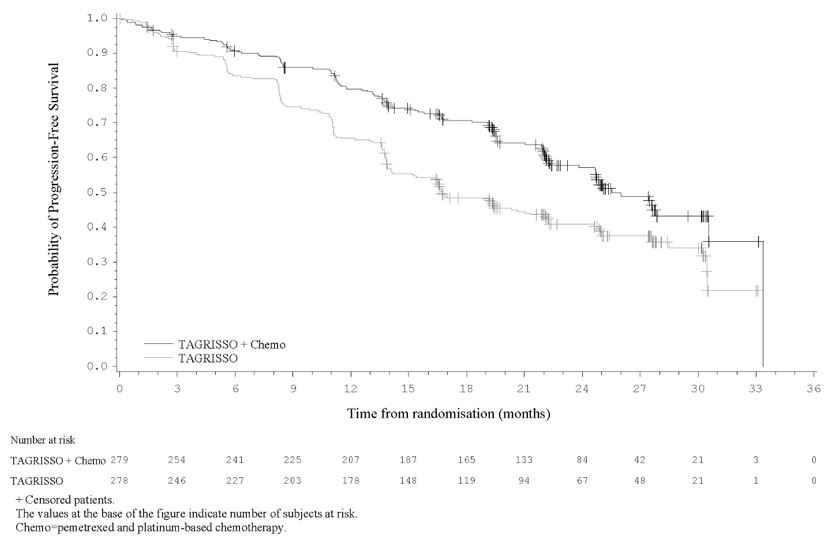
FLAURA2 demonstrated a statistically significant improvement in PFS for patients randomized to TAGRISSO in combination with pemetrexed and platinum-based chemotherapy as compared to TAGRISSO monotherapy (see Table 14 and Figure 5). While OS results were immature at the current analysis, with 45% of pre-specified deaths for the final analysis reported, no trend towards a detriment was observed.
| Efficacy Parameter | TAGRISSO with pemetrexed and platinum-based chemotherapy
(N=279) | TAGRISSO
(N=278) |
|---|---|---|
|
||
|
Progression-Free Survival (PFS)* |
||
|
PFS events (%) |
120 (43) |
166 (60) |
|
95 (34) |
158 (57) |
|
25 (9) |
8 (2.9) |
|
Median PFS in months (95% CI) |
25.5 (24.7, NE) |
16.7 (14.1, 21.3) |
|
0.62 (0.49, 0.79) |
||
|
<0.0001 |
||
|
ORR, % (95% CI) |
77 (71, 82) |
69 (63, 74) |
|
0.4 |
0.4 |
|
76 |
68 |
|
Duration of Response (DoR)# |
||
|
Median in months (95% CI) |
24.9 (22.1, NE) |
17.9 (15.2, 20.9) |
|
NE=Not Estimable; NR=Not Reached |
||
Figure 5. Kaplan-Meier Curves of PFS by Investigator Assessment in FLAURA2
All patients had available baseline brain scans reviewed by BICR using modified RECIST; 78/557 (14%) patients had CNS measurable lesions. Results of pre-specified exploratory analyses of CNS ORR and DoR by BICR are summarized in Table 15.
|
Efficacy Parameter |
CNS Measurable Lesions |
|
|
TAGRISSO with pemetrexed and platinum-based chemotherapy |
TAGRISSO |
|
|
CNS ORR, % (95% CI) |
80 (64, 91) |
76 (60, 89) |
|
48 |
16 |
|
33 |
61 |
|
Number of responders |
32 |
29 |
|
75 |
50 |
|
65 |
34 |
14.4 Previously Treated EGFR T790M Mutation-Positive Metastatic NSCLC
The efficacy of TAGRISSO was demonstrated in a randomized, multicenter open-label, active-controlled trial in patients with metastatic EGFR T790M mutation-positive NSCLC who had progressed on prior systemic therapy, including an EGFR TKI (AURA3). All patients were required to have EGFR T790M mutation-positive NSCLC identified by the cobas® EGFR Mutation Test performed in a central laboratory prior to randomization.
A total of 419 patients were randomized 2:1 to receive TAGRISSO (n=279) or platinum-based doublet chemotherapy (n=140). Randomization was stratified by ethnicity (Asian vs non-Asian). Patients in the TAGRISSO arm received TAGRISSO 80 mg orally once daily until intolerance to therapy, disease progression, or investigator determination that the patient was no longer benefiting from treatment. Patients in the chemotherapy arm received pemetrexed 500 mg/m2 with carboplatin AUC5 or pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 on Day 1 of every 21-day cycle for up to 6 cycles. Patients whose disease had not progressed after four cycles of platinum-based chemotherapy could have received pemetrexed maintenance therapy (pemetrexed 500 mg/m2 on Day 1 of every 21-day cycle).
The major efficacy outcome measure was PFS according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) by investigator assessment. Additional efficacy outcome measures included ORR, DoR, and OS. Patients randomized to the chemotherapy arm who had radiological progression according to both investigator and BICR were permitted to cross over to receive treatment with TAGRISSO.
The baseline demographic and disease characteristics of the overall trial population were: median age 62 years (range: 20-90 years), ≥75 years old (15%), female (64%), White (32%), Asian (65%), never smoker (68%), WHO performance status 0 or 1 (100%). Fifty-four percent (54%) of patients had extra-thoracic visceral metastases, including 34% with central nervous system (CNS) metastases (including 11% with measurable CNS metastases) and 23% with liver metastases. Forty-two percent (42%) of patients had metastatic bone disease.
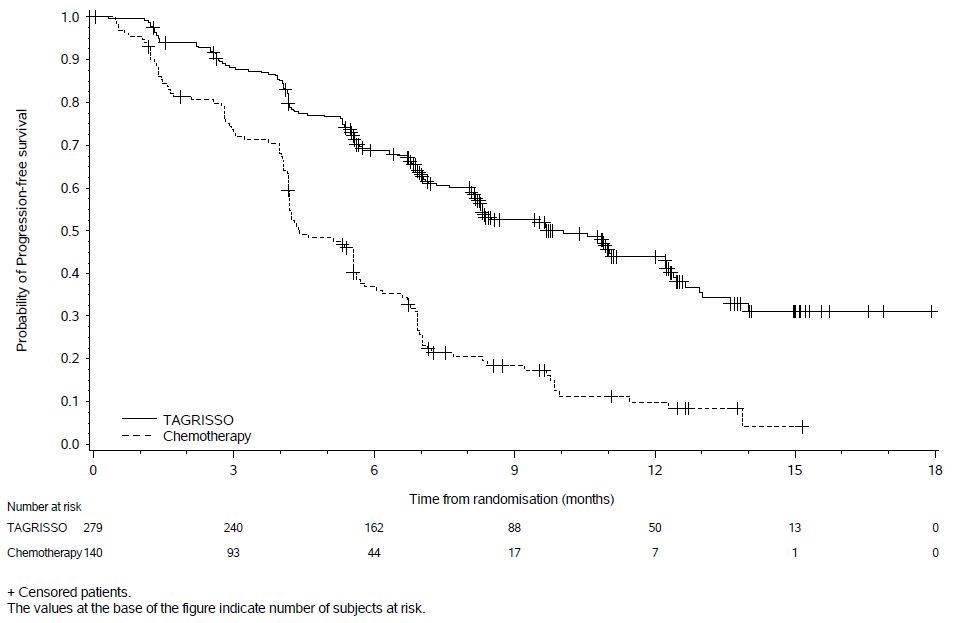
In AURA3, there was a statistically significant improvement in PFS in the patients randomized to TAGRISSO compared to chemotherapy (see Table 16 and Figure 6). No statistically significant difference was observed between the treatment arms at final OS analysis. At the time of the final OS analysis, 99 patients (71%) randomized to chemotherapy had crossed over to TAGRISSO treatment.
| Efficacy Parameter | TAGRISSO
(N=279) | Chemotherapy
(N=140) |
|
|---|---|---|---|
|
Progression-Free Survival |
|||
|
Number of events (%) |
140 (50) |
110 (79) |
|
|
Progressive disease (%) |
129 (46) |
104 (74) |
|
|
Death*(%) |
11 (4) |
6 (4) |
|
|
Median PFS in months (95% CI) |
10.1 (8.3, 12.3) |
4.4 (4.2, 5.6) |
|
|
0.30 (0.23,0.41) |
|||
|
<0.001 |
|||
|
Overall Survival | |||
|
Number of deaths (%) |
188 (67) |
93 (66) |
|
|
Median OS in months (95% CI) |
26.8 (23.5, 31.5) |
22.5 (20.2, 28.8) |
|
|
0.87 (0.67, 1.12) |
|||
|
0.277 |
|||
|
Overall Response Rate¶ |
|||
|
ORR, % (95% CI) |
65 (59, 70) |
29 (21, 37) |
|
|
Complete response, % |
1 |
1 |
|
|
Partial response, % |
63 |
27 |
|
|
<0.001 |
|||
|
Duration of Response (DoR) |
|||
|
Median in months (95% CI) |
11.0 (8.6, 12.6) |
4.2 (3.0, 5.9) |
|
Figure 6. Kaplan-Meier Curves of PFS by Investigator Assessment in AURA3
In a supportive analysis of PFS according to BICR, median PFS was 11 months in the TAGRISSO arm compared to 4.2 months in the chemotherapy arm (HR 0.28; 95% CI: 0.20, 0.38).
Of 419 patients, 205 (49%) had baseline brain scans reviewed by BICR; this included 134 (48%) patients in the TAGRISSO arm and 71 (51%) patients in the chemotherapy arm. Assessment of CNS efficacy by RECIST v1.1 was performed in the subgroup of 46/419 (11%) patients identified by BICR to have measurable CNS lesions on a baseline brain scan. Results are summarized in Table 17.
|
TAGRISSO N=30 |
Chemotherapy N=16 |
|
|
CNS ORR, % (95% CI) |
57 (37, 75) |
25 (7, 52) |
|
Complete response, % |
7 |
0 |
|
Number of responders |
17 |
4 |
|
Response Duration ≥6 months, % |
47 |
0 |
|
Response Duration ≥9 months, % |
12 |
0 |
16 HOW SUPPLIED/STORAGE AND HANDLING
80 mg tablets: beige, oval and biconvex tablet marked with “AZ 80” on one side and plain on the reverse and are available in bottles of 30 (NDC 0310-1350-30).
40 mg tablets: beige, round and biconvex tablet marked with “AZ 40” on one side and plain on the reverse and are available in bottles of 30 (NDC 0310-1349-30).
Store TAGRISSO bottles at 25°C (77°F). Excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Interstitial Lung Disease/Pneumonitis
- •
- Inform patients of the risks of severe or fatal ILD, including pneumonitis. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms [see Warnings and Precautions (5.1)].
QTc Interval Prolongation
- •
- Inform patients of symptoms that may be indicative of significant QTc prolongation including dizziness, lightheadedness, and syncope. Advise patients to report these symptoms and to inform their physician about the use of any heart or blood pressure medications [see Warnings and Precautions (5.2)].
Cardiomyopathy
- •
- Inform patients that TAGRISSO can cause cardiomyopathy. Advise patients to immediately report any signs or symptoms of heart failure to their healthcare provider [see Warnings and Precautions (5.3)].
Keratitis
- •
- Advise patients to contact their healthcare provider immediately if they develop eye symptoms (eye inflammation, lacrimation, light sensitivity, eye pain, red eye or changes in vision) [see Warnings and Precautions (5.4)].
Erythema Multiforme Major, Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis
- •
- Inform patients of signs and symptoms that may be indicative of EMM, SJS, or TEN. Advise patients to contact their healthcare provider immediately if they develop target lesions or severe blistering or peeling of skin [see Warnings and Precautions (5.5)].
Cutaneous Vasculitis
- •
- Inform patients of signs and symptoms that may be indicative of cutaneous vasculitis. Advise patients to contact their healthcare provider immediately if they develop multiple, non-blanching red papules on their forearms, lower legs, or buttocks or large hives on their trunk that do not go away within 24 hours and develop a bruised appearance [see Warnings and Precautions (5.6)].
Aplastic Anemia
- •
- Inform patients of signs and symptoms of aplastic anemia including but not limited to new or persistent fevers, bruising, bleeding, pallor, infection, tiredness or weakness. Advise patients to contact their healthcare provider immediately if signs and symptoms suggestive of aplastic anemia develop [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
- •
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider if they become pregnant or if pregnancy is suspected, while taking TAGRISSO [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
- •
- Advise females of reproductive potential to use effective contraception during treatment with TAGRISSO and for 6 weeks after the final dose [see Use in Specific Populations (8.3)].
- •
- Advise males to use effective contraception during treatment and for 4 months after the final dose of TAGRISSO [see Use in Specific Populations (8.3)].
Lactation
- •
- Advise women not to breastfeed during treatment with TAGRISSO and for 2 weeks after the final dose [see Use in Specific Populations (8.2)].
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
TAGRISSO is a registered trademark of the AstraZeneca group of companies.
©AstraZeneca 2024
|
PATIENT INFORMATION
|
||
|
What is the most important information I should know about TAGRISSO? TAGRISSO may cause serious side effects, including:
|
||
|
|
|
|
See “What are the possible side effects of TAGRISSO?” for more information about side effects. |
||
|
What is TAGRISSO? TAGRISSO is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that has certain abnormal epidermal growth factor receptor (EGFR) gene(s):
It is not known if TAGRISSO is safe and effective in children. |
||
|
Before taking TAGRISSO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. Especially tell your healthcare provider if you take a heart or blood pressure medicine. |
||
|
How should I take TAGRISSO?
|
||
|
What are the possible side effects of TAGRISSO? TAGRISSO may cause serious side effects:
The most common side effects of TAGRISSO when given alone include: |
||
|
|
|
|
The most common side effects of TAGRISSO in combination with pemetrexed and platinum-based chemotherapy include: |
||
|
|
|
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of TAGRISSO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store TAGRISSO?
|
||
|
General information about the safe and effective use of TAGRISSO. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TAGRISSO for a condition for which it was not prescribed. Do not give TAGRISSO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TAGRISSO that is written for health professionals. |
||
|
What are the ingredients in TAGRISSO? Active ingredient: osimertinib Inactive ingredients: mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and sodium stearyl fumarate. Tablet coating contains: polyvinyl alcohol, titanium dioxide, macrogol 3350, talc, ferric oxide yellow, ferric oxide red and ferric oxide black. Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850 © AstraZeneca 2024 For more information, go to www.Tagrisso.com or call 1-800-236-9933. |
||
- This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 02/2024