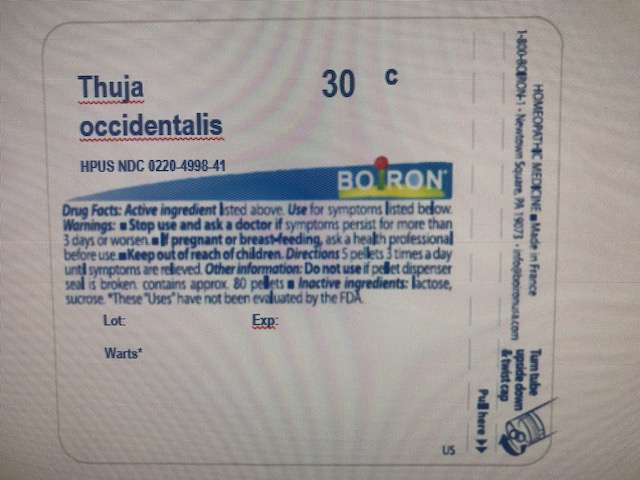
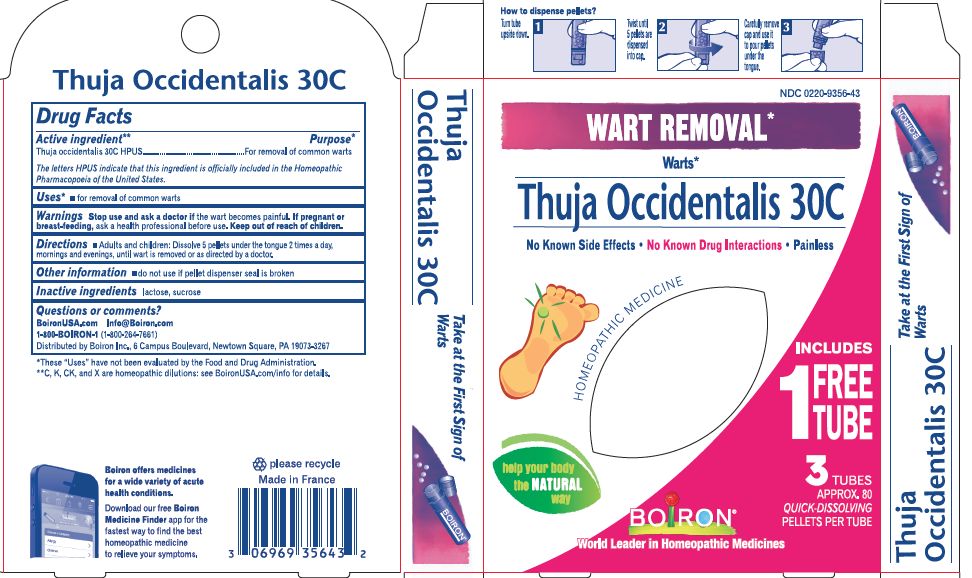
THUJA OCCIDENTALIS 30C HPUS (0.443 mg) (in each pellet)**
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Adults and children: Dissolve 5 pellets under the tongue 2 times a day, mornings and evenings, until wart is removed or as directed by a doctor.
Boironusa.com
info@boiron.com
1-800-BOIRON-1
1-800-264-7661
Distributed by Boiron Inc.
Newtown Square, PA 19073
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
Turn tube upside down, twist until 5 pellets are dispensed, Remove cap and pour pellets under the tongue
3 Tubes, Approx. 80 Pellets Each, Total 240 Pellets, 16 Doses per tube
Pain-Free Wart Removal*
No Known Drug Interactions
Common Warts, Plantar Warts*
Wart Removal*