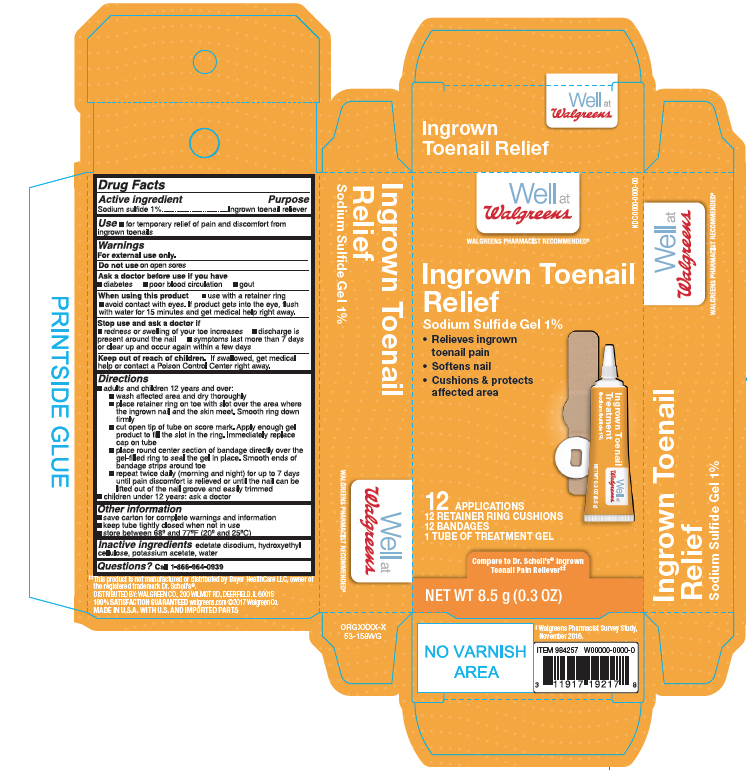
SODIUM SUFIDE- ingrown toenail pain relief kit gel
Walgreens Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Sodium Sufide 1%
Purpose
Ingrown Toenail Pain Reliever
Use
- for the temporary releif of pain and discomfort from ingrown toenails
For external use only.
Do not use
on open sores.
Ask a doctor before use if you have
- diabetes
- poor blood circulation
- gout
When using this product
- use with a retainer ring
- avoid contact with eyes. If prodcut gets into the eye, flush with water for 15 minutes and get medical help reight away.
Stop use and ask a doctor if
- redness or swelling of your toe increases
- discharge is present around the nail
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children 12 yers and over:
- wash affected area and dry thoroghly
- place retainer ring on toe with slot over the area where the ingrown nail and the skin meet. Smooth ring down firmly
- cut open tip of the tube on score mark. Apply enough gel prodcut to fill the slot in the ring. Immeciately replace cap on tube
- place round center section of bandage directly over the gel- filled ring to seal the gel in place. Smooth ends of bandage strips around toe
- repeat twice daily (morning and night) for up to 7 days until pain discomfort is relieved or until the nail can be lifted out of the nail groove and easliy trimmed
Children under 12 years: ask a doctor
Other Information
- save carton for complete warnings and information
- keep tube tightly closed when not in use
- store between 68o anf 77oF (20o and 25oC)
Inactive Ingredient
edetate disodium, hydroxyethyl cellulose, pottasium acetate, water
Questions?
Call 1-866-964-0939
Principal Display Panel
Well at Walgreens
Ingrown Toenail Relief
Sodium Sulfide Gel 1%
- Relieves ingrown toenail pain
- Softens nail
- Cushions & protects afftected area
12APPLICATIONS
12 RETAINER RING CUSHIONS
12 BANDAGES
1 TUBE OF TREATMENT GEL
NET WT 8.5 g(0.3 OZ)