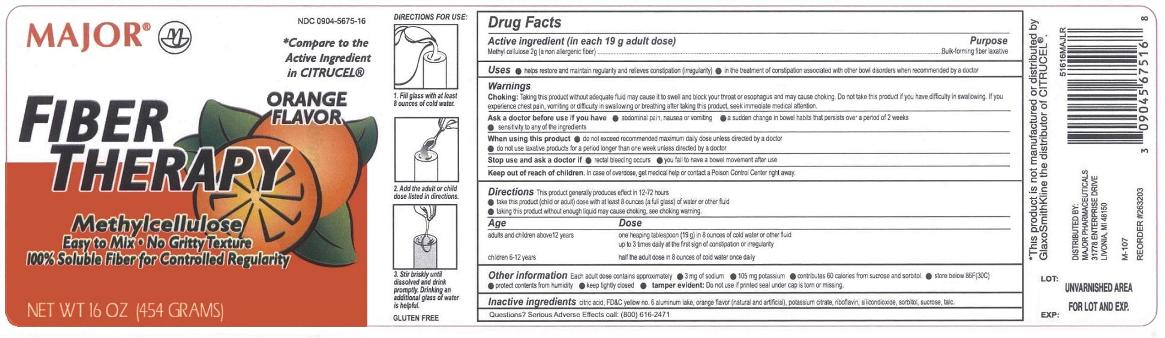
Active ingredient
(in each 19g adult dose/ rounded tablespoon)
Methylcellulose 2g (a non-allergenic fiber)
Uses
- •
- relieves constipation (irregularity)
- •
- helps to restore and maintain regularity
- •
- for treating bowel disorders when recommended by a doctor
- •
- generally provide effect in 12-72 hours
Warnings
CHOKING: taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Ask a doctor before use if you have
- •
- abdominal pain, nausea or vomiting
- •
- a sudden change in bowel habits that persists over a period of 2 weeks
- •
- sensitivity to any of the ingredients
Stop use and ask a doctor if
- •
- you have rectal bleeding
- •
- you fail to have a bowl movement after use.
- •
- These could be signs of a serious condition.
Directions
- •
- MIX THIS PRODUCT (CHILD OR ADULT DOSE) WITH AT LEAST 8 OUNCES (A FULL GLASS) OF WATER OR OTHER FLUID. TAKING THIS PRODUCT WITHOUT ENOUGH LIQUID MAY CAUSE CHOKING. SEE CHOKING WARNING
- •
- use product at the first sign of constipation or irregularity
- •
- put one dose in a full glass of cold water
- •
- stir briskly and drink promptly
- •
- drinking another glass of water is helpful
|
Age |
Dose |
|
adults & children above 12 years of age and over |
One rounded tablespoon. (19 g) in 8 ounces of water fluid up to 3 times daily at the first sign of constipation or irregularity |
|
children 6 - 12 years of age Children under 6 years of age |
half the adult dose in 8 ounces of water once daily. ask a doctor |
Other information
- •
- Each adult dose contains approximately
- •
- 3mg of sodium
- •
- 105mg potassium
- •
- contributes 60 calories from sucrose and sorbitol
- •
- store at room temperature
- •
- protect contents from humidity
- •
- keep tightly closed
Tamper Evident: Do not use if printed seal under cap is torn or missing
Inactive ingredients
citric acid, FD&C yellow 6 aluminum lake, maltodextrin, flavor (natural and artificial), potassium citrate, , silica, sucrose,
Questions or comments?
call toll-free 1-800-616-2471
*This product is not manufactured or distributed by GlaxoSmithKline the distributor of CITRUCEL®
Distributed by:
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152, USA
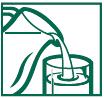
DIRECTIONS FOR USE
1. Fill glass with at least 8 ounces of cold water.
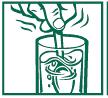
2. Add the adult or child dose listed in the directions. 3. Stir briskly until dissolved and drink promptly. Drinking an additional glass of water is helpful.