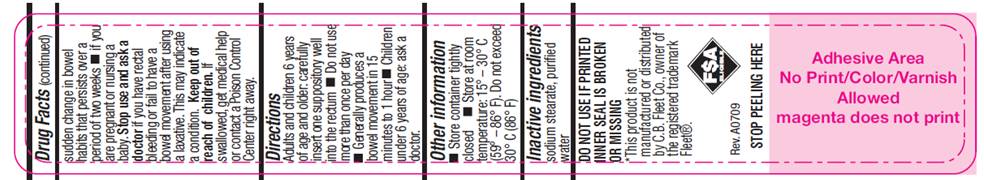
WARNINGS
For rectal use only. May cause rectal discomfort or a burning sensation.
Do not use
- more than one per day
- for a period of longer than one week unless directed by a doctor
- laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
- if seal under product lid is damaged, missing or broken.
Ask a doctor before use
- if you have noticed a sudden change in bowel habits that persist over a period of two weeks
- if you are pregnant or nursing a baby
DIRECTIONS
- Adults and children 6 years of age and older: carefully insert one suppository well into the rectum
- Do not use more than once per day
- Generally produces a bowel movement in 15 minutes to 1 hour
- Children under 6 years of age: ask a doctor.
OTHER INFORMATION
- Store container tightly closed
- Store at room temperature: 15°- 30° C (59° - 86° F). Do not exceed 30° C (86° F).
PACKAGE LABEL
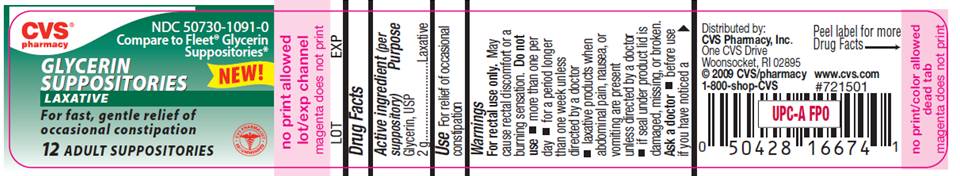
CVS pharmacyNDC 50730-1091-0
Compare to Fleet Glycerin Suppositories
NEW!
GLYCERIN
SUPPOSITORIES
LAXATIVE
For fast, gentle relief of
occasional constipation
12 ADULT SUPPOSITORIES
Distributed by CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
©CVS/pharmacy
1-800-shop-CVS
www.cvs.com
#721501
INNER SEAL IS BROKEN
OR MISSING
This product is not
manufactured or distributed
by C.B.Fleet co., owner of
the registered trademark
Fleet®.