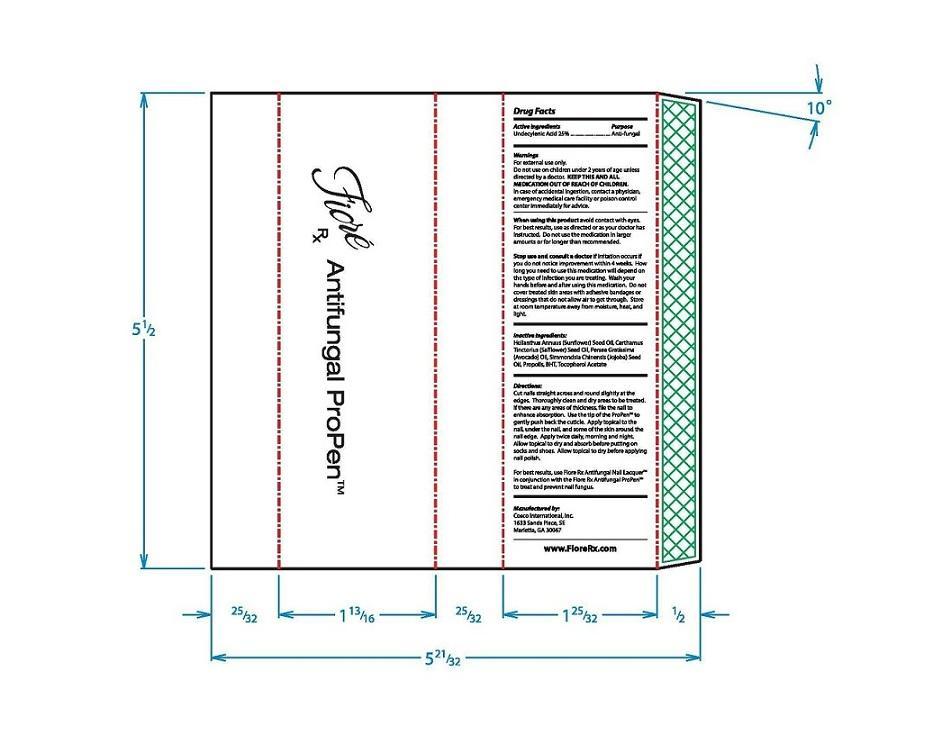
Do not use on children under 2 years of age unless directed by a doctor.
KEEP THIS AND ALL MEDICATION OUT OF REACH OF CHILDREN.In case of accidental ingestion, contact a physician, emergency medical care facility or poison control center immediately for advice.
Directions:
Cut nail straight across and round slightly at the edges. Throughly clean and dry areas to be treated. If there are any ares of thickness, file the nail to enhance absorption. Use the tip of the ProPen™ to gently push back the cuticle. Apply topical to the nail, under the nail, and some of the skin around the nail edge. Apply twice daily, morning and night. Allow topical to dry and absorb before putting on socks and shoes. Allow topical to dry before applying nail polish.
For best results, use Fiore Rx Antifungal Nail Lacquer™ in conjuction with the Fiore Rx Antifungal ProPen™ to treat and prevent nail fungus.