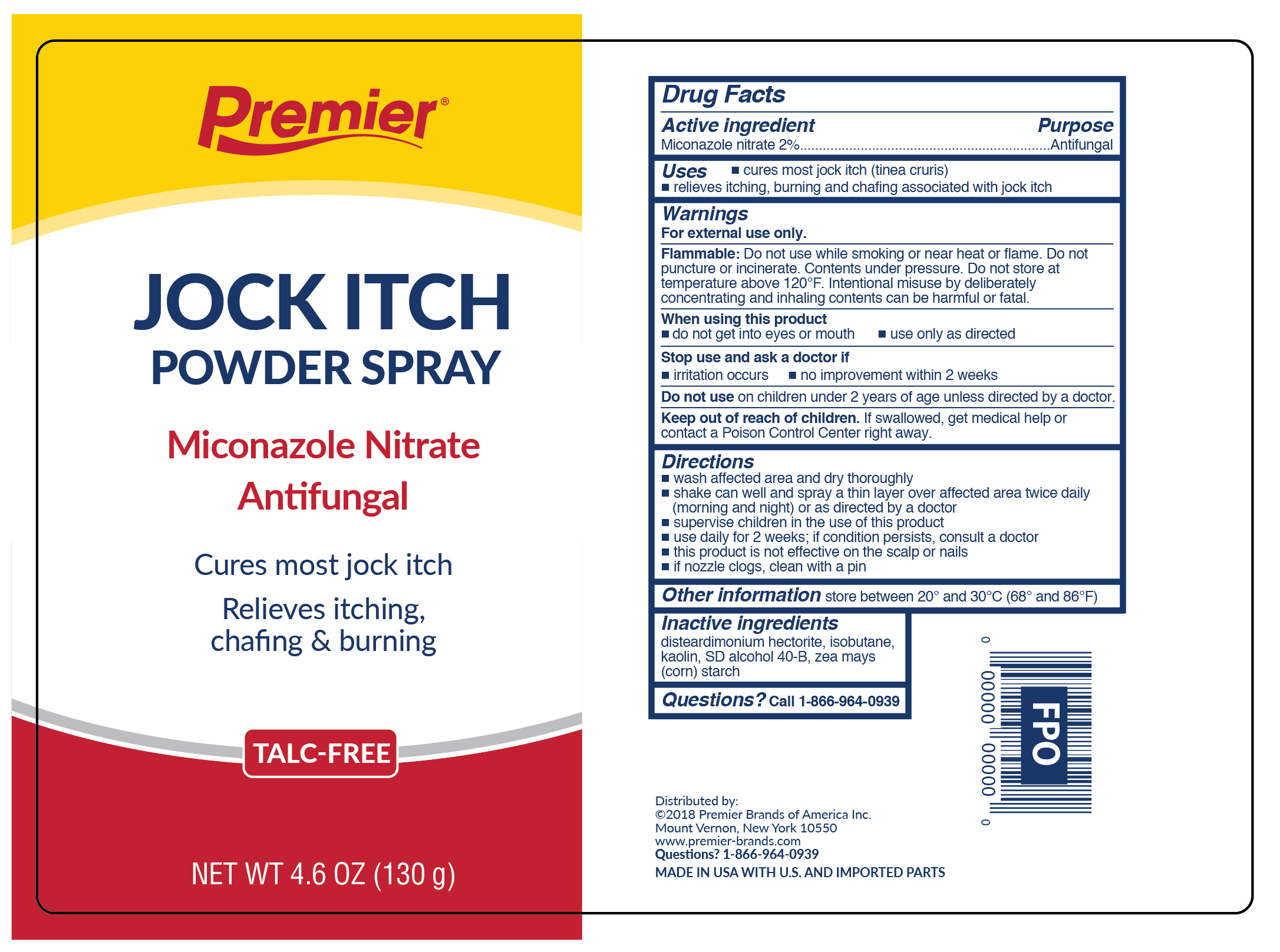
Active ingredient
Miconazole nitrate 2%
Uses
- cures most jock itch (tinea cruris)
- relieves symptoms of jock itch including itching, burning and chafing associated with jock itch
Warnings
For external use only.
Flammable:
Do not use while smoking or near heat or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperature above 120ºF.
When using this product
- do not get into eyes or mouth
- use only as directed. Intentional misuse by deliberately concentrating and inhaling contents cans be harmful or fatal.
Stop use and ask a doctor if
- irritation occurs
- no improvement within 2 weeks for jock itch
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Do not use on children under 2 years of age. Do not use for diaper rash. Do not use on children under 2 years of age unless directed by a doctor.
Directions
- wash affected area and dry thoroughly
- shake can well and spray a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- use daily for 2 weeks. If conditions persist, consult a doctor
- this product is not effective on scalp or nails
- in case of clogging, clear nozzle under running water
Inactive ingredient
Disteardimonium Hectorite, Isobutane, Kaolin, SD Alcohol 40-B, Zea Mays (Corn) Starch
Questions?
Call 1-866-964-0939
Principal Display Panel
Premier
Jock Itch Powder Spray
Miconazole Nitrate Antifungal
Cures most jock itch
Relieves itching, chafing and burning
TALC-FREE
NET WT 4.6 OZ (130g)