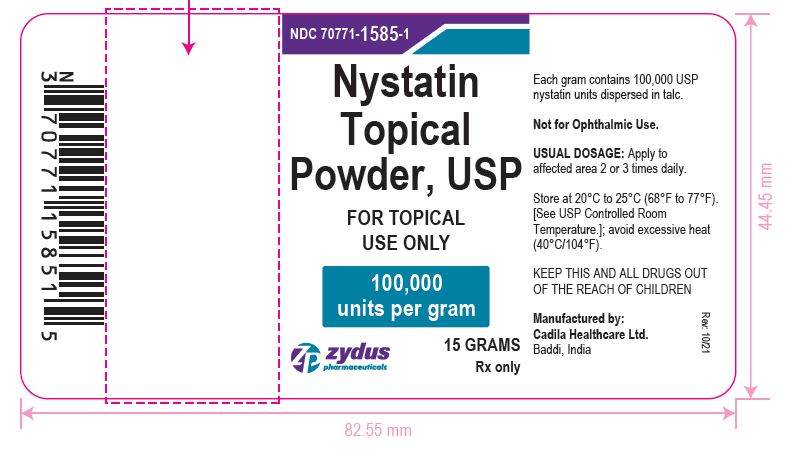
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nystatin Topical Powder, USP
FOR TOPICAL USE ONLY
100,000 units per gram
Rx Only
15 GRAMS
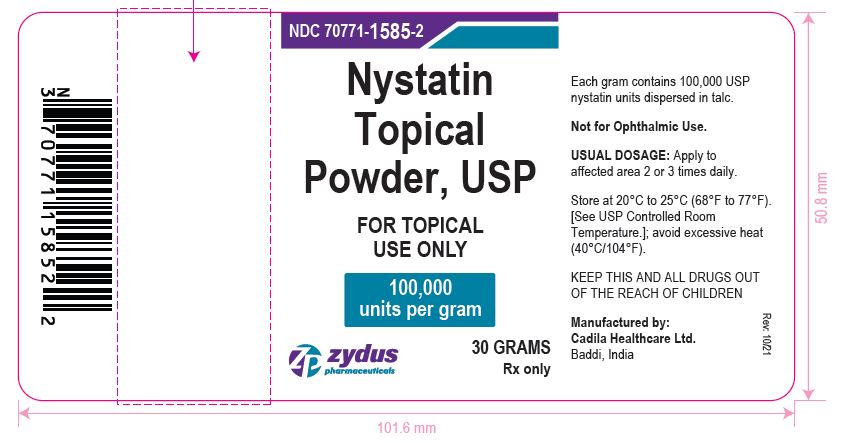
Nystatin Topical Powder, USP
FOR TOPICAL USE ONLY
100,000 units per gram
Rx Only
30 GRAMS
Nystatin Topical Powder, USP
FOR TOPICAL USE ONLY
100,000 units per gram
Rx Only
60 GRAMS
